Research Team Led by Professor Wei-Guo Zhu Publishes Groundbreaking Study in Nature Revealing Novel Mechanism of Chromatin Decompaction During DNA Damage Response
Supported by Shenzhen University's “2035 High-Level Research Initiative” and the Medical School’s Structured Research Program”, a research team led by Professor Wei-Guo Zhu from the School of Basic Medical Sciences, Shenzhen University Medical School, and the Carson International Cancer Center has published a landmark study in Nature. The article, titled “Histone H1 deamidation facilitates chromatin relaxation for DNA repair”, was released on April 16, 2025, and unveils a previously unknown mechanism by which linker histone H1 deamidation drives chromatin relaxation during the DNA damage response.
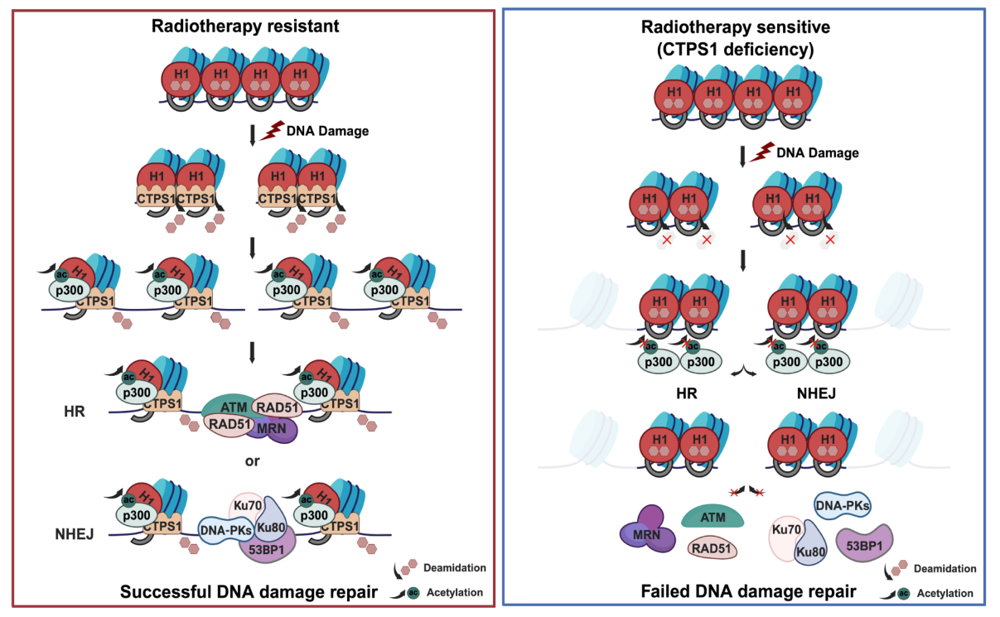
The team discovered that following DNA double-strand breaks, asparagine residues 76 and 77 (Asn76/77) on histone H1 undergo deamidation catalyzed by cytidine triphosphate synthase 1 (CTPS1). This post-translational modification promotes acetylation of the adjacent lysine 75 (Lys75), with histone acetyltransferase p300 displaying a marked preference for the deamidated form of H1—indicating that deamidation primes subsequent acetylation. Structural analyses revealed that Lys75 lies within the globular domain of histone H1, a critical DNA-binding interface. Acetylation at this site weakens H1-DNA interactions, triggering nucleosome destabilization and chromatin relaxation, and thereby facilitating the recruitment of DNA repair machinery to damaged sites.
The study proposes a model in which the Lys75–Asn76–Asn77 triad functions as a molecular “fastener” regulating chromatin compaction. These findings not only shed light on fundamental chromatin remodeling processes in response to genotoxic stress but also lay the groundwork for the development of precision therapeutics targeting tumor resistance to radiotherapy and chemotherapy.
This publication in Nature represents a significant milestone in Shenzhen University's pursuit of global academic excellence and highlights the institution’s growing impact in the field of basic medical research.
Professor Wei-Guo Zhu is the corresponding author of the study. Yuan Tian, Ting-Ting Feng, and Jun Zhang (all from Shenzhen University) are co-first authors. The study also involved collaborations with Ming Tang (Tongji University Shanghai First Maternity and Infant Hospital), Chao-Hua Liu (Fudan University Shanghai Cancer Hospital), Yu-Xin Shu (Wannan Medical College), and Yu Zhang (Department of Medical Genetics, Peking University Health Science Center).
URL:https://doi.org/10.1038/s41586-025-08835-0









用户登录
还没有账号?
立即注册