Genome Instability & Disease Volume 4. Issue 6 简介
1. The role of protein arginine methylation 5 in DNA damage repair and cancer therapy | Qikun Gao, Ziyi Liu, Jinyang Liu, Xuyang Yan, Junfei Dai, Zixuan Zhang, Rongxiao Li, Shiva Basnet & Changzheng Du
Protein methyltransferase (PRMT) is an important factor that helps to regulate protein function and stability. PRMT has been implicated in promoting carcinogenic processes such as tumor proliferation, invasion, and immune escape, as well as DNA damage repair via different signaling pathways. In this review, Professor Changzheng Du and colleges from the Southern University of Science and Technology, China, summarize the specific biological functions of PRMT5 in cancer, DNA damage repair, and genome stability. They then discuss the latest progress made in developing and applying PRMT5 inhibitors to cancer treatment.
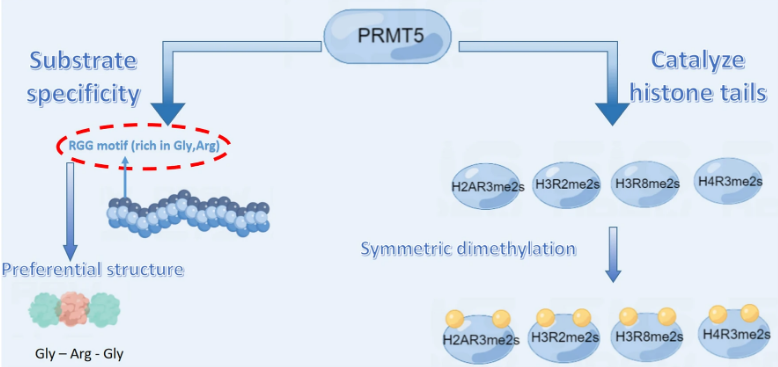
Fig.1 Substrate specificity of PRMT5.
蛋白质甲基转移酶(PRMT)是调节蛋白质功能和稳定性的重要因子,通过不同的信号通路在促进肿瘤增殖、侵袭性、免疫逃逸和DNA损伤修复等致癌过程中发挥着关键作用。在这篇综述中,来自南方科技大学的杜长征教授团队向我们总结了蛋白质甲基转移酶5(PRMT5)在癌症DNA损伤修复和维持基因组稳定性中的生物学功能,并总结了PRMT5抑制剂在癌症治疗中的最新进展。

杜长征教授
全文链接:https://link.springer.com/article/10.1007/s42764-023-00111-7
2. Chemotherapy-induced neuronal DNA damage: an intriguing toolbox to elucidate DNA repair mechanisms in the brain | Aishwarya Babu & Madhusoodanan Urulangodi
Neuronal DNA damage is associated with various neurological diseases, including Alzheimer, Parkinson, and Huntington disease. In this review, Dr. Aishwarya Babu and Madhusoodanan Urulangodi from the Sree Chitra Tirnal Institute of Medical Science and Technology (SCTIMST), India, explain the layers of DNA repair and regulation involved in maintaining genomic integrity in neurons. They start by introducing us to the known mechanisms underlying the repair of neuronal DNA damage. They then focus on the types of DNA damage and the ensuing repair that occurs in the brains of cancer patients. Finally, they discuss the long-term effects of chemotherapy on brain function.
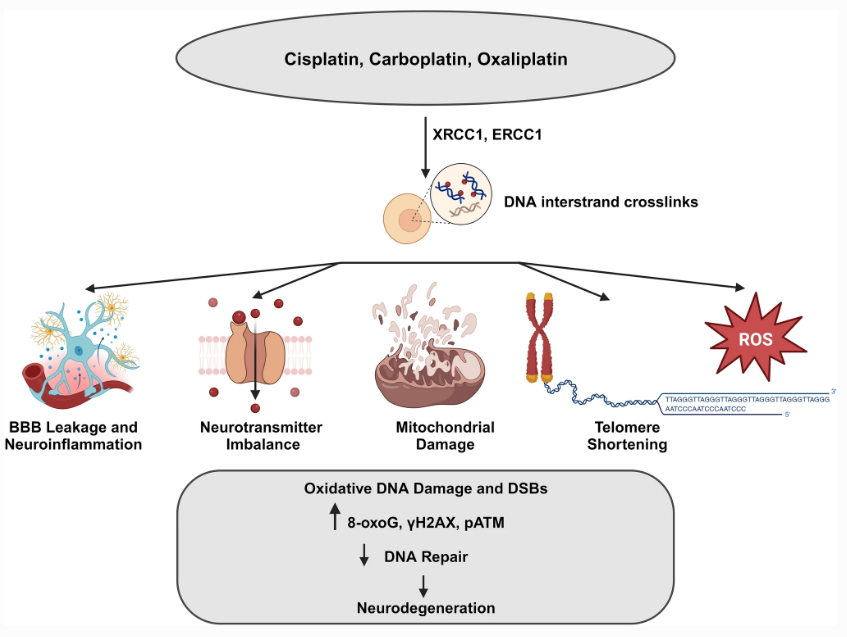
Fig.2 Schematic representation of direct and indirect actions of chemotherapeutic drugs on brain.
神经元DNA损伤与阿尔茨海默病,帕金森病,亨廷顿舞蹈症等多种神经疾病密切相关,来自印度Sree Chitra Tirunal医学科学与技术研究所(SCTIMST)的Aishwarya Babu 和 Madhusoodanan Urulangodi博士向我们介绍了神经元DNA损伤修复的各种热点问题,尤其是化疗对癌症患者大脑DNA损伤及修复机制的改变以及大脑功能的长期影响,帮助我们更好地了解神经元中维持基因组完整性所涉及的DNA修复机制及调控。

Madhusoodanan Urulangodi 博士
全文链接:https://link.springer.com/article/10.1007/s42764-023-00110-8
3. An integrated pan-cancer analysis of leucine-rich repeat containing protein 59: a potential biomarker for prognostic and immunotherapy | Meiqi Zeng, Xia Wang, Xiaona Wang, Yuning Zhang, Zhenguang Ying, Lixin Xia, Feng Gao, Xianxiong Chen, Kin Yip Tam, Long Xu & Ou Sha
LRRC59 is a protein that is rich in leucine repeat sequences, and binds to ribosomes located on the endoplasmic reticulum and nuclear membrane. Professor Ou Sha and colleagues from the Medical Department of Shenzhen University, China, performed a multi-omics data analysis of multiple databases to investigate the expression of LRRC59 in human tumors and its correlation with clinical prognosis, gene set enrichment, mutation status, and immune infiltration. They found evidence to support that LRRC59 is a potential cancer biomarker that can be leveraged in making a cancer diagnosis and prognosis, but also in cancer immunotherapies.
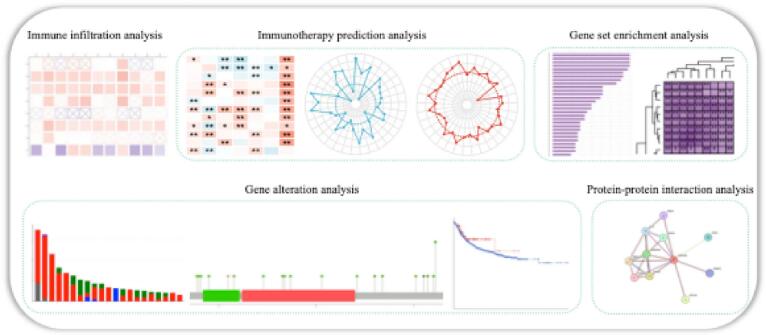
Fig. 3 Protein–protein interaction between LRRC59 and other proteins.
富含亮氨酸重复序列的蛋白质59(LRRC59)是一种位于内质网和核膜上的核糖体结合蛋白,深圳大学医学部的沙鸥教授及同事利用多个数据库和R包进行了多组学数据分析,研究LRRC59在人类肿瘤中的表达及其与癌症临床预后、基因集富集、突变状态和免疫浸润的相关性。该研究表明LRRC59是一种潜在的癌症生物标志物,可应用于诊断,预后,以及大多数的癌症免疫治疗。

沙鸥教授
全文链接: https://link.springer.com/article/10.1007/s42764-023-00113-5
4. PDHE1α in DNA damage repair: a critical chromatin acetylation regulator | Bingsong Huang, Yuping Chen & Jian Yuan
Professor Wei-Guo Zhu and colleagues from Shenzhen University recently published an article in Nature Structural & Molecular Biology, reporting the mechanism of how chromatin-related pyruvate dehydrogenase E1 α (PDHE1α) locally produces acetyl CoA to reshape the chromatin environment near double strand breaks, and promote DNA damage repair. Here, Professor Jian Yuan and colleagues from TongJi University, China, provide their commentary on the findings published by Zhu et al. They discuss the importance of these new data, namely in resolving the mystery of the origins of nuclear acetyl CoA. They also explain how the findings have helped to further delineate the complex process of DNA damage repair and chromatin acetylation regulation, and open new avenues for cancer intervention and treatment.
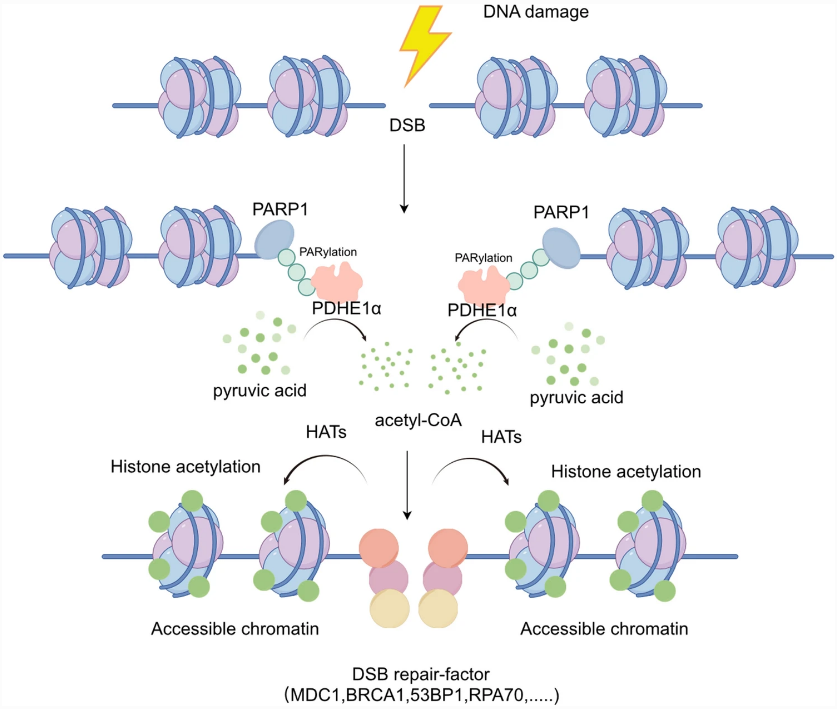
Fig. 4 After DNA damage, PDHE1α is recruited to double-strand break (DSB) sites in a PARylation-dependent manner mediated by PARP1.
深圳大学的朱卫国团队近期在《Nature Structural & Molecular Biology》报道了染色质相关丙酮酸脱氢酶E1α(PDHE1α)局部产生乙酰辅酶a以重塑DSBs附近的染色质环境并促进其修复的机制。同济大学的袁健教授对此进行点评,指出该文章解决了细胞核乙酰辅酶a的起源之谜,揭示了DNA损伤修复和染色质乙酰化调控的复杂过程,为癌症干预治疗开辟了新的参考。

袁健教授
全文链接:https://link.springer.com/article/10.1007/s42764-023-00112-6
5. H3K9me3 asymmetry: epigenetic choreography in DNA replication for genomic stability | Wei-Guo Zhu
Parental histones must be precisely allocated and assembled into the chromatin of daughter cells during the DNA replication stage of mitosis. Doing so ensures the accurate transmission of epigenetic information between parental and daughter cells. Recently, Professor Zhiguo Zhang and colleagues from Columbia University reported in Nature, a unique distribution pattern of H3K9me3 during DNA replication, and explained this phenomenon from a molecular perspective. Here, Professor Wei-Guo Zhu from Shenzhen University, China, provides his perspective on these intriguing findings, pointing out the scientific significance of the study but also highlighting the remaining issues that still need to be solved, such as: How is H3K9me3 asymmetry resolved outside of S phase? What is the role of SETDB1 or other methyltransferases in this process? How does H3K9me3 inhibit L1 expression due to the asymmetry of the lead chain? Addressing these intriguing questions will help us continue to develop our understanding of the mechanisms of epigenetic inheritance.
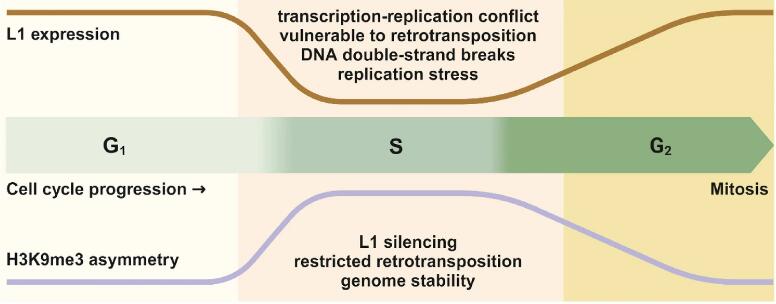
Fig. 3 Protein–protein interaction between LRRC59 and other proteins.
有丝分裂DNA复制过程中,亲代组蛋白如何分配组装到子代染色质, 确保表观遗传信息在亲子代间的准确传递,一直以来未得到确切的验证。最近,来自哥伦比亚大学肿瘤遗传研究的张志国教授团队发表在《自然》杂志上的文章报道了H3K9me3在DNA复制过程中的独特分布模式,从分子机制上解释了这一科学问题。深圳大学的朱卫国教授对此进行点评,指出该研究的科学意义,并指出还有一些问题尚待解决,比如H3K9me3不对称性是如何在S相外解决的,SETDB1或其他甲基转移酶在这一过程中的作用是什么?H3K9me3对前导链的不对称性是如何抑制L1表达的?这些问题的解答,将帮助我们对表观遗传有更深的理解。

朱卫国教授
全文链接: https://link.springer.com/article/10.1007/s42764-023-00117-1








用户登录
还没有账号?
立即注册