Primary microcephaly with an unstable genome
Review Article Published: 02 September 2020
Shibin Xu, Xingxuan Wu, Bin Peng, Sheng-Li Cao & Xingzhi Xu
Genome Instability & Disease 1,235–264(2020)
Abstract
Autosomal recessive primary microcephaly, also known as MCPH, is a rare genetic condition where infants are born with small heads and brains. The causes of MCPH are often unknown or unclear. To date, 25 genes have been found to be associated with MCPH. Most of these genes serve similar roles in maintaining genome stability, being associated with centrosome and spindle function, chromosome dynamics, cell cycle regulation, cell division, brain development, neurogenesis, and/or the DNA damage response. In this review, we classify MCPH-associated genes based on their known functions, and propose potential novel functions of MCPH genes in DNA replication and/or the DNA replication stress response, and tumorigenesis. This classification provides a novel perspective on the underlying causes of MCPH and a comprehensive reference for future research.
Introduction
Microcephaly, derived from the Greek words for “small” (μικρό) and “head” (κεφάλι), is a rare neurological birth defect characterized by a significantly smaller head than normal. For a clinical diagnosis of microcephaly, the occipital frontal circumference diameter must be three standard deviations smaller than that of other infants of the same age and sex; while, the length of the femur must be within two standard deviations of the normal value. Affected infants typically have a smaller brain volume than normal but lack any major brain structure abnormalities. The condition is often, but not always, accompanied by intellectual disabilities. There is no curative treatment, and severe cases often require lifelong management. Thus, understanding the underlying causative factors of microcephaly is imperative to develop novel treatments and/or improve present disease management strategies.
Microcephaly can be classified as either ‘primary’ or ‘secondary’. In primary microcephaly, less brain tissue than normal develops during pregnancy, and this can be caused by congenital infections, maternal alcohol consumption, drug use during pregnancy, chromosomal aberrations and/or incorrect mitotic spindle alignment caused by damaged DNA (Akbariazar et al. 2013; Barbelanne and Tsang 2014; Naveed et al. 2018; COWIE 1960; Woods 2004; Faheem et al. 2015). However, in secondary microcephaly, the brain tissue develops normally during pregnancy but is stymied following birth. This outcome might be due to oxygen deprivation, brain injury or infection. This form of microcephaly is characterized by decreased numbers of dendrites and synaptic connections (Abdel-Hamid et al. 2016).
Although the cause of any single case is often unknown, a range of both genetic and environmental factors have been linked to the disease. This review focuses on the genes involved in autosomal primary microcephaly (MCPH). Previous articles have identified dozens of genes linked to MCPH, and have been highlighted that these genes are involved in vital activities including cell cycle regulation, DNA repair, and mitotic spindle function. Here, we classify these MCPH-associated genes based on their functions and summarize the evidence supporting these, to provide a comprehensive reference for researchers in the field. These genes are summarized in Table 1, and their functions are discussed in further detail below. In light of the previous research indicating that dysfunction of certain MCPH-associated genes is associated with DNA replication, the DNA replication stress response, and tumorigenesis, we summarize the links between alterations in key MCPH-associated genes and various cancers (Venkatesh and Suresh 2014; Brüning-Richardson et al. 2011; Zhang et al. 2013a, b).
Table 1 MCPH-associated genes
| Gene name | Gene symbol | Gene function | References |
|---|---|---|---|
| Microcephalin 1 | MCPH1 | Cell cycle checkpoint regulation, chromosome condensation maturation, and CHK1 and BRCA1 expression in the DNA damage response signaling pathway | Trimborn et al. (2004); Xu et al. (2004); Liu et al. (2017) |
| WD repeat domain 62 | MCPH2/WDR62 | Stabilization of spindle orientation, interactions between the kinetochore and spindle microtubule, centriole duplication | Kodani et al. (2015); Bilgüvar et al. (2010); Bogoyevitch et al. (2012); Yu et al. (2010) |
| CDK5 regulatory subunit-associated protein 2 | MCPH3/CDK5RAP2 | Microtubule organization, centriole duplication, spindle organization and spindle checkpoint function | Kodani et al. (2015); Barr et al. (2010); Zhang et al. (2009) |
| Kinetochore scaffold 1 | MCPH4/KNL1 | Microtubule attachment to the centromere, spindle-assembly checkpoint (SAC) activation during mitosis | Genin et al. (2012); Kiyomitsu et al. (2011) |
| Assembly factor for spindle microtubules | MCPH5/ASPM | Centriole duplication, spindle orientation, spindle organization, kinetochore association | Gai et al. (2016); Hsu et al. (2019) |
| Centromere protein J | MCPH6/CENPJ | Centriole duplication | Zheng et al. (2016); Tang et al. (2009) |
| SCL/TAL1 interrupting locus protein | MCPH7/STIL | Centriole assembly and duplication, cell cycle | Patwardhan et al. (2018) |
| Centrosomal protein 135 | MCPH8/CEP135 | Maintaining the structure and organization of the centrosome and of microtubules | Lin et al. (2013); Singh et al. (2014) |
| Centrosomal protein 152 | MCPH9/CEP152 | Centriole duplication | Lee et al. (2018); Sonnen et al. (2013) |
| Zinc finger protein 335 | MCPH10/ZNF335 | Chromatin remodeling complex formation regulating neuronal gene expression and cell fate | Yang et al. (2012) |
| Polyhomeotic homolog 1 | MCPH11/PHC1 | Cell cycle regulation, DNA damage response signaling | Awad et al. (2013) |
| Cyclin dependent kinase 6 | MCPH12/CDK6 | Cell cycle regulation, spindle organization, kinetochore association | Lucas et al. (2004); Beukelaers et al. (2011) |
| Centromere protein E | MCPH13/CENPE | Microtubule organization, spindle organization, kinetochore association | Mirzaa et al. (2014) |
| Spindle assembly abnormal protein 6 homolog | MCPH14/SASS6 | Centriole formation via procentriole complex formation | Keller et al. (2014); Lin et al. (2013) |
| Major facilitator superfamily domain containing 2A | MCPH15/MFSD2A | Blood–brain barrier transmembrane protein, fatty acid transportation | Harel et al. (2018); Guemez-Gamboa et al. (2015) |
| Ankyrin repeat and LEM domain containing 2 | MCPH16/ANKLE2 | Neural precursor cells proliferation during initial brain development, central nervous system (CNS) biogenesis | Asencio et al. (2012); Kaufmann et al. (2016) |
| Citron rho-interacting serine/threonine kinase | MCPH17/CIT | Spindle orientation, DNA repair, midbody formation for cell division | Li et al. (2016); D'Avino (2017) |
| WD repeat and FYVE domain containing 3 | MCPH18/WDFY3 | Selective self-breakdown of macromolecules, cell signaling regulator | Simonsen et al. (2004); Kadir et al. (2016) |
| COPI coat complex subunit beta 2 | MCPH19/COPB2 | Component of the Golgi and vesicular trafficking system | DiStasio et al. (2017) |
| Kinesin family member 14 | MCPH20/KIF14 | Mitotic spindle dynamics, cell division | Gruneberg et al. (2006) |
| Non-SMC condensin I complex subunit D2 | MCPH21/NCAPD2 | Compaction of interphase chromatin into mitotic chromosomes | Martin et al. (2016) |
| Non-SMC condensin II complex subunit D3 | MCPH22/NCAPD3 | Compaction of interphase chromatin into mitotic chromosomes | Martin et al. (2016) |
| Non-SMC condensin I complex subunit H | MCPH23/NCAPH | Compaction of interphase chromatin into mitotic chromosomes | Martin et al. (2016) |
| Nucleoporin 37 microtubule associated protein 11 | MCPH24/NUP37 | Nuclear pore complex | Loïodice et al. (2004) |
| Microtubule associated protein 11 | MCPH25/MAP11 | Mitotic spindle dynamics | Perez et al. (2019) |
Centrosome and mitotic spindle function-associated pathogenic genes
A large percentage of MCPH genes is related to the mitotic spindle or centrosome, and located at the centrosome or spindle (Bond and Woods 2006; Nigg and Raff 2009). Centrosomes are a fundamental part of the cytoskeleton, and serve as the microtubule organizing centers (MTOC) of the cell. The centrosomes establish bipolar spindles during mitosis, which ensure the precise, symmetrical separation of chromosomes. The centrosomes also modulate other cellular processes, including cell cycle progression, cell shape, cilia assembly, DNA damage repair and genomic stability (Alieva and Uzbekov 2008; Debec et al. 2010; Nigg and Raff 2009). They also maintain cell polarity and provide a framework for the directional transport of other organelles (Bond et al. 2005; Barbelanne and Tsang 2014). Most MCPH-associated genes are also involved in the control of mitotic spindle orientation during neural development, and multiple proteins encoded by MCPH-associated genes are associated with centrosome function (Miyamoto et al. 2017). The mechanisms controlling the orientation of the cleavage plane should be operated very precisely, due to its key role in affecting cell fate determination during neurogenesis (Zhong and Chia 2008). A common centrosome- or spindle-related mechanism regulating brain size has been proposed, including MCPH1-3, MCPH5-9, MCPH12, MCPH14 and MCPH25.
Microcephalin (MCPH1)
MCPH1/BRIT1, located on chromosome 8p23, encodes the microcephalin protein. This protein consists of three breast cancer carboxyl terminal (BCRT) domains, which are common to proteins involved in DDR signaling and cell cycle control (Liu et al. 2016). MCPH1 localizes to the centrosomes throughout the cell cycle, and is involved in the cell cycle regulatory functions (Jeffers et al. 2008). MCPH1 depletion leads to centrosomal anomalies, mitotic spindle misalignment and delayed cytokinesis and polyploidy, promoting genomic instability and malignant transformation in U2OS cells (Rai et al. 2008).
MCPH1 is the first pathogenic gene reported to cause primary microcephaly. It was identified after an analysis of patients from two consanguineous Pakistani families with primary microcephaly symptoms associated with premature chromosome condensation (Neitzel et al. 2002). Subsequently, a large deletion mutation covering the first six exons of the microcephalin gene was also reported in patients from an Iranian family with mild microcephaly (Garshasbi et al. 2006). MCPH1-deficient progenitors have a character of multiple spindle poles, chromosome misalignment, and lagging chromosomes (Gruber et al. 2011). The microcephaly phenotype has also been demonstrated by Gruber et al. in a MCPH1-null mutant mouse model. In addition, several MCPH1-deficient mouse models showed reduced thickness of the neocortex at birth (Trimborn et al. 2010; Chen et al. 2013; Liang et al. 2010; Gruber et al. 2011). Besides, MCPH1-deficient progenitors increased the production of early-born neurons, which comprise the deep layers (IV–VI), and decreased the late-born neurons, which produce the thinner outer cortex layer (II–III) (Zhou et al. 2013). It is a specific effect of MCPH1 on brain size, rather than an effect on overall body size, confirmed by CNS-specific deletion of MCPH1 in mice (Gruber 2011).
Early mitotic entry at the G2–M transition was discovered in MCPH1-null cells due to deregulated activation of centrosomal cyclin B–CDK1 and premature CDK1 activation (Gruber et al. 2011). Recently, MCPH1 was shown to be involved in cell cycle regulation by interacting with ubiquitin E3 ligase SCFβTrCP2 or APC/CCdh1 complexes, which cooperate to regulate cyclin regulator CDC25–CDK1 to ensure proper cell cycle transition (Liu et al. 2017; Choudhury et al. 2016). The cell fate decision of neuroprogenitors is closely linked to the cell cycle progression (Cheffer et al. 2013). Premature CDK1 activation induces early mitotic entry and mitotic spindle orientation disruption in neuroprogenitors. This uncoupling of the cell cycle from the centrosome cycle shifts the division plane of neuroprogenitors from symmetric to asymmetric(Gruber et al. 2011). In mouse model, absence of MCPH1 in neuronal progenitor cells disrupted the balance of self-renewal and differentiation and led to premature depletion of the neuronal progenitor cell pool (Gruber 2011). Another report showed loss of MCPH1 in mice primarily affects the generation of upper-layer neurons linking to complexity of cognitive functions (Zhou et al. 2013; Molnar et al. 2006).
WD repeat domain 62 (WDR62/MCPH2)
The WDR62 gene contains 32 exons and is located in the MCPH2 candidate region on chromosome 19q13.12. It encodes a 1523-amino acid protein with multiple WD40 repeats, which are ~ 40-amino acid-long structural motifs that normally end in a tryptophan–aspartic acid dipeptide (Cherkaoui Jaouad et al. 2018). A WDR62 null was identified as the second most common cause of MCPH due to the discovery of homozygous missense and frame-shifting mutations in seven MCPH Arab, Pakistani and Caucasian families (Adeline K. Nicholas et al. 2010). Patients with MCPH typically lack severe architectonical brain abnormalities, but patients with WDR62 mutations often present with a wide spectrum of cortical malformations, including cortical thickening, polymicrogyria, simplified gyral patterns, pachygyria, schizencephaly, heterotopias and corpus callosum abnormalities. Some patients also have evidence of lissencephaly, cerebellar hypoplasia and hippocampal dysmorphia (Bilgüvar et al. 2010). WDR62 mutations, c.1313G>A and c.4241dupT, affect WDR62 expression at the spindle poles during mitosis cells and result in a cerebral cortex lamination defect (Nicholas et al. 2010). Another report, with a cohort including a patient with a particularly severe microcephalic phenotype, found that the underlying cause was a novel, homozygous splicing variant of MCPH2 (c.3335+1G>C) in the WD repeat domain 62 gene (GenBank accession number: NM_005682.5). Brain magnetic resonance imaging was performed at 3 years of age and revealed extensive areas of polymicrogyria in the right frontal lobe, and abnormal gyration with a blurring of the gray–white matter in the left parietooccipital cortex. This patient also had an additional variant in the tubulin cofactor D (TBCD) gene, which has a crucial role in cortical development (Bilgüvar et al. 2010).
WDR62 is a prominent spindle pole protein that serves an important function in mitotic spindle stabilization during mitosis. It accumulates at the spindle pole in a cell cycle-dependent manner, from late prophase until the metaphase–anaphase transition. Phosphorylation of WDR62 during mitotic progression coincides with spindle pole localization (Bogoyevitch et al. 2012). WDR62 depletion results in defective metaphase spindles, characterized by centrosome displacement. Mutations to MCPH2/WDR62 result in a randomized spindle orientation that is caused by the defective assembly of astral microtubules (Bogoyevitch et al. 2012). It was observed in HeLa cells and lymphocytes of MCPH2-mutant patients that WDR62 depletion resulted in disruption of spindle pole structure, fragmented centrosomes and even mitotic delay (Farag et al. 2013).
WDR62 is identified as a substrate of Polo-like kinase 1 (PLK1), a mitotic kinase essential for maintaining spindle orientation through the development of astral microtubules. WDR62 is phosphorylated at Serine 897 by PLK1, and this phosphorylation event at the spindle poles promotes astral microtubule assembly and stabilization of spindle orientation. The PLK1–WDR62 axis at the spindle poles might maintain MTOC activity and control kinetochore–spindle microtubule interaction (Miyamoto et al. 2017). WDR62 has been also reported to play a key role in centriole duplication and partitioning in dividing cells. WDR62 interacts with the centriole satellite protein OFD1 and FOPNL interacting protein (OFIP/MOONRAKER) involved in the process of centriole localization of cyclin-dependent kinase 2 (CDK2) to promote duplication (Kodani et al. 2015). In addition, WDR62 also functions with ASPM for the elongation of daughter centrioles (Jayaraman et al. 2016).
WDR62, as a scaffold protein, is involved in the c-Jun N-terminal kinase (JNK) signaling pathway to control self-renewal and differentiation of neural progenitor cells during brain development by forming a complex with MAP kinase kinases (MKKs) 4 and 7, and JNKs (Bogoyevitch et al. 2012; Cohen-Katsenelson et al. 2011; Xu et al. 2018a). Loss of JNK signaling resulted in constitutive WDR62 localization to microtubules irrespective of cell cycle stage, while phosphorylation of WDR62 at T1053, mediated by JNK1, negatively regulates microtubule association (Lim et al. 2015). WDR62 also been reported to couple with Aurora kinase A (AURKA) for the spindle localization of WDR62 during mitosis and then affect neuroblast number (Lim et al. 2016, 2017). Spindle misorientation induced by WDR62 depletion changes cell fate determination during neurogenesis since proper spindle orientation can ensure the neuroepithelial stem cells (NESCs) dividing asymmetrically, producing precursor cells of differentiated neurons (Noatynska et al. 2012; Wang et al. 2009). Thus, WDR62 controls neurogenesis and brain size through the regulation of JNK activity (Xu et al. 2018a, b).
Another report shows that WDR62 and AURKA co-knockdown specifically in the glial lineage, altered brain growth (Lim et al. 2017). Silencing of AURKA in glia significantly decreased glial number and brain volume and co-depletion of WDR62 impair brain growth by increasing glial cell death (Lim et al. 2015, 2017). What is more, WDR62 depletion in the glia could increase neural stem death and significantly reduced the neuroblast pool.
In addition, mice deficient in ASPM and WDR62 affected the apical complex components, and disrupted the fate switch from apical to basal progenitors (Jayaraman et al. 2016). Loss of function in WDR62 and ASPM leads to severe centriole loss which has a positive relationship with the degree of cortical thinning and precocious neuroprogenitor differentiation in the mouse embryo, supporting centriole biogenesis defects as underlying microcephaly in WDR62-null mice.
CDK5 regulatory subunit-associated protein 2 (CDK5RAP2/MCPH3)
CDK5RAP2 is a centrosomal protein expressed by various tissues and contains a γ-tubulin-association domain and a fly ortholog centrosomin that recruits the γ-tubulin ring complex to the centrosome (Issa et al. 2013a). The importance of this gene to the cell cycle was demonstrated that the centrosomes were separated from the mitotic spindles in CDK5RAP2-knockout cells, and mitotic orientation was altered in mice with a mutated form of the gene (Ohta et al. 2015; Lizarraga et al. 2010). The increased number of abnormal mitotic figures and aneupolar spindles observed in these mice suggests that CDK5RAP2 serves a critical role in spindle and centrosome function during neurogenesis.
CDK5RAP2 is an EB1-binding protein that forms a CDK5RAP2–EB1 complex, regulating microtubule dynamics and stability. EB1 proteins are highly conserved and are localized to cytoplasmic microtubule tips (Fong et al. 2009; Akhmanova and Steinmetz 2008; Vaughan 2005). Similarly, CDK5RAP2 is concentrated at the distal tips of microtubules. RNA interference-mediated silencing of CDK5RAP2 affects the dynamic behavior of microtubules, and may target the tips of EB1-associated growth microtubules to regulate microtubule dynamics (Vaughan 2005; Fong et al. 2009).
CDK5RAP2 is also required for normal germ cell cycle progression, as confirmed by the labeling of heterozygous pregnant mice with two different thymidine analogs: 5-iodo-20-deoxyuridine and 5-bromo-20-deoxyuridine (BrdU) (Zaqout et al. 2017). Depleted or otherwise nonfunctional CDK5RAP2 seems to result in mitotic delay, and the decreased number of neuronal progenitors observed in Cdk5rap2an/an mice correlates with an increased mitotic index, suggesting delayed mitosis (Lizarraga et al. 2010). The Cdk5rap2an/an mutant allele possesses an in-frame deletion of exon 4 in Cdk5rap2 in Hertwig’s anemia mice. Thus, it seems that CDK5RAP2 is associated with the G2/M transition of the cell cycle.
The implications of CDK5RAP2 deficiency, and the resultant mitotic delay and germ cells depletion, are far-reaching. For example, a severe reduction in testis volume due to a massive loss of germ cells have been reported in a CDK5RAP2-deficient mouse model (Zaqout et al. 2017). CDK5RAP2 also serves an important role during physiological eye development, as demonstrated by the eye malformations observed in Cdk5rap2an/an-mutant mice. These malformations range from cataracts, reduced retinal thickness, monocular or binocular miniaturization (microphthalmia) to complete binocular deletion (anophthalmia). The number of phosphor-histone H3-positive mitotic cells and apoptotic cells with activated cleaved caspase-3 are increased in Cdk5rap2an/an mice (Zaqout et al. 2020). Furthermore, and perhaps most pertinently, Cdk5rap2an/an animals have a smaller cerebral cortex than wild-type animals due to an overall reduction of neuronal layers, caused by decreased numbers of progenitor cells. It is somewhat confusing that anemia has not been reported in the few known human patients with CDK5RAP2 mutations. However, in Cdk5rap2an/an mice, both microcephaly and anemia are variable and strain dependent, and it is possible that similar modifiers also affect penetrance in human (Lizarraga et al. 2010). Homozygous Cdk5rap2an mice with microcephaly exhibit a small cerebral cortex that correlates with multipolar spindles, misalignment of spindles in neural precursors, and loss of neural progenitors (Lizarraga et al. 2010).
Research in two boys of Italian descent with primary microcephaly identified the novel nonsense homozygous CDK5RAP2 mutation (c.4441C>T) leading to a nonsense mutation p.Arg1481* (Issa et al. 2013b). The resulting CDK5RAP2 protein was deficient in mitotic spindle regulation and γ-tubulin localization to the centrosome for duplication. This finding suggests that spindle defects and disruption of centrosome integrity function indispensably in microcephaly development.
Abnormal spindle-like, microcephaly-associated protein (ASPM/MCPH5)
ASPM maps to chromosome 1q31, consists of 28 exons, and is critical for mitotic spindle function. Notably, mutations in the ASPM gene are the most common cause of MCPH, occurring in ~ 40% of both consanguineous and non-consanguineous families without any genotype/phenotype correlations (Tan et al. 2014; Nicholas et al. 2009; Abdel-Hamid et al. 2016). The first ASPM clone was constructed by Bond et al. (2002). The full-length ASPM protein has a molecular mass of ~ 410 kD, contains 3477 amino acids and includes four domains: the putative microtubule-binding domain, the calponin-homology domain, the multiple IQ calmodulin-binding domain, and the carboxyl terminal region. A novel mutation in the donor splicing site of exon 8 was detected, and was found to alter the transcription of ASPM and thus functionally impair the gene product (Nicholas et al. 2009; Tan et al. 2014; Fujimori et al. 2014).
A functional link exists between ASPM and MPCH17/CIT—a protein whose loss is responsible for severe microcephaly in mammals. CIT controls mitotic spindle orientation in developing mouse and fly brains by promoting the nucleation and stability of astral microtubules. An in situ proximity ligation assay revealed that CIT interacts with both cytoplasmic and spindle-associated ASPM, suggesting that ASPM might control spindle function via its interaction with CIT. It is possible that low dose of microtubule-stabilizing drugs could alleviate the spindle orientation phenotype produced by their knockdown (Gai et al. 2016).
ASPM also maintains spindle position during mitosis in neuroendocrine cells, ensuring that the precise cleavage plane orientation required for symmetrical cell division is present. ASPM knockdown via RNA interference severely affects centrosome localization in M-phase NE cells, and detachment of the centrosomes from sister chromatids suggesting that ASPM deletion might occur during sister chromatid separation (Fish et al. 2006). However, ASPM knockdown does not inhibit middle-stage mitosis. Labeling of the mitotic marker phospho-histone H3 and BrdU incorporation assays revealed that ASPM prevents entry into the S phase of the cell cycle. Disruption of ASPM causes mitotic arrest and apoptotic cell death during early embryogenesis (Fish et al. 2006; Fujimori et al. 2008; Kim et al. 2011). ASPM is important for the symmetric proliferative division of neuroepithelial cells during brain development, and the decreasing progenitor pool caused by increasing asymmetric divisions in ASPM-deficient neural progenitor cells is the primary mechanism underlying microcephaly (Fish et al. 2006).
Overall brain volume is reduced by ~ 50% in patients with ASPM mutations, including homozygous c.7782_7783delGA and c.6651_6654delAACA, with the volume of white matter being decreased by > 50%. The subcortical gray matter and cerebellum seem to be less affected. The hippocampus volume is relatively preserved compared with that of the neocortical areas in both hemispheres in patients with ASPM mutations. Furthermore, the gyrification index is also reduced in these patients, which affects different cortical regions to varying extents. For example, patients with ASPM mutations display preserved amnesic capacity, but mildly reduced or borderline intellectual abilities (Passemard et al. 2016).
This phenotype was confirmed in the fetal brains of ASPM knockout mice, which also showed a decreased cerebrum volume that was associated with a thinner, deep cortical layer VI and an aberrant expression pattern of layer-specific transcription factors including Tbr1 and Satb2 (Nicholas et al. 2009). Decreased ASPM expression was also detected following X-ray and carbon ion beam treatments in both human and murine tissue culture cells, and similar phenomena were noted in fetal mouse brains, particularly in the periventricular region of the day 12 telencephalon, and in the neurospheres (Fujimori et al. 2008).
Centromere protein J (CENPJ/MCPH6)
CENPJ, which is also known as centrosomal-P4.1-associated protein (CPAP), is a key component of the centrosome, and its localization in the microtubules suggests that it might be involved in the process of microtubule nucleation (Hung et al. 2000, 2004).
CENPJ has been reported to bind to pericentriolar complexes including γ-tubulin, pericentrion and CDK5RAP2 (Chou et al. 2016). dSAS-4, which is a CENPJ Drosophila homolog, can localize to the centrioles and pericentriolar material (PCM), which induces the production of multiple MTOCs and can directly or indirectly recruit PCM proteins (Nigg and Raff 2009). dSAS-4 can also protect from dispersion of the pericentriolar proteins, centrosomin, and γ-tubulin, which can cohere PCM proteins and maintain spindle pole integrity during mitosis (Lecland et al. 2013). Depleted CENPJ inhibits centrosome duplication; whereas, overexpressed CENPJ results in the formation of elongated procentriole-like structures, which is required for procentriole elongation (Tang et al. 2009). CENPJ has a key role in controlling centriole length during S/G2 phase, its overexpression of CENPJ in human cells enhances the accumulation of centriolar tubulin, leading to centrioles of strikingly increased length (Schmidt et al. 2009). In sum, CENPJ positively functions in controlling centriole length during S/G2 phase.
An siRNA-based phenotypic screen revealed that CENPJ is required for the proper formation of centrioles, along with SASS6, CP110, CEP135 and γ-tubulin. Of note, SASS6 associates with nascent procentrioles; whereas, CEP135 and CENPJ form a core structure within the proximal lumen of centrioles (Gönczy 2012; Jana et al. 2014). This function of CENPJ and SASS6 was further confirmed by analyses conducted in invertebrates, connecting CENPJ to centriole duplication (Kleylein-Sohn et al. 2007; Basto et al. 2006; Peel et al. 2007; Rodrigues-Martins et al. 2007a, b). Except for centrosome duplication defects, downregulation of CENPJ in HeLa cells leads to spindle malformations and modifies the orientation of the cleavage plane (Kitagawa et al. 2011). In addition, the absence of CENPJ in embryos results in an elongated centriole and a short delay in the prometaphase caused by the absence of centrioles triggers p53-dependent apoptosis in the rapidly dividing cells of the mouse embryo (Bazzi and Anderson 2014).
CENPJ is not only necessary for centriole biogenesis, but also functions as a regulator of ciliary disintegration. In neural progenitor cells, CENPJ depletion results in the lengthening of cilia and their abnormal disintegration. The absence of CENPJ in radial glial cells leads to the incomplete division of cortical cells, decreased cell proliferation and increased cell apoptosis in developing mice, leading to primary microcephaly (Ding et al. 2019).
Bond et al. identified a homozygous nonsense mutation (E1235V) in the CENPJ gene in each of three MCPH6 families using a positional cloning strategy. They showed that CENPJ can control the number of neurons generated by neural precursor cells (Bond et al. 2005). Conditional mutant mice for CENPJ/Sas-4 exhibit neurogenesis defects in microcephaly that might arise from the depletion of centrosomes and subsequent loss of primary cilia (Insolera et al. 2014). CENPJ disruption in cortical progenitors leads to a centrosome formation abnormality that results in perturbed spindle orientation during mitosis (Garcez et al. 2015). CENPJ is required to promote microtubule destabilization, acting a key role in proper nuclear–centrosome coupling and nucleokinesis during late neurogenesis, which is another crucial step of cortical development. Furthermore, CENPJ depletion in radial glial cells leads to reduced cell proliferation, and increased cell apoptosis in the developing mouse cerebrum cortex, resulting in microcephaly (Ding et al. 2019).
SCL/TAL1-interrupting locus protein (STIL/MCPH7)
STIL was first identified in T-cell leukemia through cDNA fusions, which contains 17 exons and encodes 1,288 amino acids (Gustafsson et al. 2018). The asymmetrical localization of STIL to the bases of procentrioles is important for proper centriole replication, and heterozygous mutations can cause lengthening of the centrioles and, thus, microcephaly (Tang et al. 2011). STIL levels differ substantially across cell cycle stages: being low during G1 phase, gradually increasing during and after G1–S transition, and finally declining during metaphase–anaphase transition. The serine/threonine protein kinase 4 (PLK4) is related to the Polo-kinase family of serine/threonine kinases and initiates centriole formation (Habedanck et al. 2005). Knockdown of PLK4 or STIL prolongs mitosis, resulting in severe mitotic defects. It is important to consider the mechanisms underlying cell self-renewal and differentiation when studying cell cycle regulation. For example, higher p53 protein levels are observed after PLK4/STIL depletion, which results in a lack of centrosomes; this effect in turn causes p53 upregulation. This upregulation is even more marked than that induced by retinoic acid (Renzova et al. 2018).
Centriole duplication starts with PLK4 activation. PLK4 can phosphorylate STIL and recruits STIL at the base of the parental centriole (Renzova et al. 2018; Arquint and Nigg 2016). Then SASS6, a coiled-coil protein that self-assembles into a ninefold symmetry that gives it a cartwheel shape, cooperates with PLK4 and phosphorylates STIL on the outside wall of the centriole and functions as the core module for centriole duplication to initiate the formation of a new procentriole (Arquint and Nigg 2016). CENPJ can also be recruited to the centriole along with the core module for the beginning of the cartwheel assembly.
Three different homozygous STIL mutations linked to the MCPH7 locus have been identified in patients with microcephaly: IIS-17, IIS-28, and IIS-3, all of which have been predicted to cause a truncated STIL protein (Kumar et al. 2008). In human cells, the absence of functional STIL blocks centriole replication, while overexpression of STIL leads to excessive centriole formation (Cristofoli et al. 2017). Microcephaly in MCPH7 patients can result from a decrease in STIL levels; this finding was supported by rescue experiments in U2OS cells, where the pGly717Glu mutation induces a reduced but non-null activity of STIL on centriole duplication (Mouden et al. 2015).
Centrosomal protein 135 (CEP135/MCPH8)
CEP135 encodes a 135-kDa protein that is implicated in various centrosome functions, including early centriole assembly, replication, biogenesis and formation. A CEP135 deletion mutation (c.970delC), resulting in a premature termination codon, was identified in patients from one Pakistan family (Hussain et al. 2012).
As mentioned, CEP135 and PLK4, SASS6, STIL, CENPJ and CEP120 are involved in cartwheel formation and procentriole assembly (Kleylein-Sohn et al. 2007; Chang et al. 2016). CEP135 acts as a scaffold protein during early centriole biogenesis, targets centriole satellite proteins such as PCM1, Afadin- and alpha-actinin-binding protein (SSX2IP) and CEP290, and mediates the recruitment of a regulator of spindle anchoring at the centrosome (SSX2IP–WRAP73 complex) to the centriole. CEP135 is also connected to SASS6 and CENPJ via its involvement in cartwheel function and the outer microtubules (Lin et al. 2013). Depletion of CEP135 not only perturbs the centriolar localization of CENPJ, but also blocks CENPJ-induced centriole elongation. Thus, CEP135 disruption alters the numbers of microtubule triplets and shorter centrioles, which have a negative effect on centriole assembly. Centrosome amplification, affecting mitotic progression and mitotic spindle orientation, usually leads to multipolar spindles linking to the loss of progenitor cells; this results in reduced neuron production, which might be the cause of microcephaly. This finding was supported by research in Drosophila neuroblasts in which mutating the centriolar protein BLD10 (the fly ortholog of CEP135) resulted in failed establishment of centrosome asymmetry through shedding of Polo from the mother centrosome. Centrosome asymmetry has been also implicated in stem cell fate maintenance in neuroblasts (Singh et al. 2014).
Centrosomal protein 152 (CEP152/MCPH9)
CEP152 is one of several MCPH proteins that interact with their corresponding centriolar satellite components, as follows: CDK5RAP2 with Sperm-associated antigen 5 (SPAG5), CEP72 and CEP152 with CEP131, WDR62 with MOONRAKER, and CEP63 and CEP9 with Coiled-coil domain-containing protein 14 (CCDC14) (Kodani et al. 2015). CEP152, CEP135 and STIL have been suggested to facilitate centriole duplication by recruiting proteins to the centrosome, including PLK4 (Kodani et al. 2015). PLK4, a key regulator for centriole duplication, has recently been associated with Seckel syndrome, which is a heterogeneous, autosomal recessive disorder characterized by microcephaly (Kalay et al. 2011). CEP152, together with CEP192, recruits PLK4 to the centrosome and induces nascent centriole assembly (Sonnen et al. 2013). There are some evidences to suggest that CEP152 is also involved in Seckel syndrome, and that it regulates the DNA damage pathway (Sonnen et al. 2013; Hatch et al. 2010; Dinçer et al. 2017). CEP152 is also known to interact with CEP63 (a centrosomal protein of 63 kDa) to ensure efficient centriole duplication by promoting the accumulation of essential centriole duplication factors upstream of SASS6 recruitment and procentriole formation (Brown et al. 2013). CEP63 and CEP152 self-assemble into a pericentriolar cylindrical architecture and this event is critical for the orderly recruitment of PLK4 for centriole duplication (Wei et al. 2020). CEP57 (a centrosomal protein of 57 kDa) also interacts with the CEP63–CEP152 complex, which is required for proper CEP152 recruitment and centriole duplication (Wei et al. 2020). Furthermore, CEP152, WDR62 and CDK5RAP2 also interact with each other; if all are depleted, centriole duplication is inhibited (Kodani et al. 2015). CEP152 modulates procentriole formation by promoting the centrosomal accumulation of CENPJ and PLK4 (Hatch et al. 2010; Cizmecioglu et al. 2010).
CEP152 might represent a mammalian ortholog of the Drosophila gene asterless; the mutations of which affect mitosis in the fly (Guernsey et al. 2010; Varmark et al. 2007; Blachon et al. 2008). CEP152 is also expressed in the embryonic mouse brain, similar to other MCPH genes, as confirmed by RT-PCR. Mutations in the centrosomal protein CEP152 have been identified families with primary microcephaly linked to MCPH4 (Guernsey et al. 2010). As mentioned, centrosome deficiency is usually linked to the loss of progenitor cells, which results in reduced neuron production—a potential cause of microcephaly.
Cyclin-dependent kinase 6 (CDK6/MCPH12)
CDK6, encoded by a gene on chromosome 7q21.2, is a serine/threonine-protein kinase that regulates major cell cycle events and differentiation of various eukaryotic cells. CDK6 phosphorylates retinoblastoma-associated protein (pRB/RB1) to promote the G1-to-S phase transition during the cell cycle (Meyerson and Harlow 1994). Previous studies have revealed that CDK6 inhibits the proliferation of human mammary epithelial cells, and prevents differentiation of leukemic cells and osteoblast cells (Matushansky et al. 2003; Ogasawara et al. 2004; Lucas et al. 2004). CDK6 disruption results in disorganized mitotic spindles, distorted microtubules, supernumerary centrosomes and reduced cell proliferation. An increased distance between the centrosomes and the nucleus and impaired cell polarity was also observed in primary fibroblasts. In neuronal precursor cells, a CDK6 deficiency promotes the asymmetric division of neurons and depletion of progenitor cells, which could be a key mechanism underlying primary microcephaly (Hussain et al. 2013).
A recurrent homozygous missense mutation (c.589G>A) in the CDK6 gene was identified in 10 affected members of a Pakistani family with MCPH12 (Hussain et al. 2013). The mutation, which was found by homozygosity mapping, candidate gene analysis, and whole-exome sequencing, segregated with the disorder in the family. CDK6 in patient cells failed to localize at the centrosome during mitosis. Patient cells showed disorganized mitotic spindles, misshapen nuclei, supernumerary centrosomes, reduced cell proliferation and impaired cell motility and polarity compared to controls.
Highly expression of CDK6 was observed in cortical progenitor and striatal stem cells, CDK6 disruption prolonged G1-phase and inhibited entry into S-phase by the Rb regulatory pathway (Ferguson et al. 2000). Juvenile mice lacking CDK6 showed similar patern of precursor cells in the subventricular zone and subgranular zone of the dentate gyrus with prolonged G1 phase, premature cell cycle exit, and attenuated proliferation (Beukelaers et al. 2011). It was hypothesized that microcephaly caused by a CDK6 mutation is a consequence of increased neural stem/progenitor apoptosis and decreased cell proliferation due to reduced retinoblastoma phosphorylation phenomena in the mutant fibroblasts (Muhammad S. Hussain et al. 2013). This hypothesis was supported by G1-phase lengthening in mouse neuroepithelial cells, which led to premature differentiation. These effects explain the decreased proliferation associated with CDK6 disruption (Calegari and Huttner 2003).
Spindle assembly abnormal protein 6 homolog (SASS6/MCPH14)
SASS6 is a highly conserved, coiled-coil protein that is required for centriole formation in the cells of species as evolutionarily distant as C. elegans and humans. Abnormal SASS6 protein expression leads to disordered polarity of spindles, suggesting that it is necessary for normal progression of the centrosome duplication cycle (Leidel et al. 2005). SASS6 is required for procentriole formation and to prevent additional procentriole formation, ensuring that each centriole seeds the formation of a single procentriole per cell cycle (Strnad et al. 2007). During centriole formation, SASS6 self-assembles into tetramers and, with the activation of PLK4 on the surface of the parental centriole, a complex of CEP135, STIL, SASS6 and CENPJ initiates procentriole formation (Gopalakrishnan et al. 2010). In recent years, a module comprising protein PLK4, STIL and SASS6 has been shown to have a key role in centriole duplication: depletion of any one of these three proteins blocks centriole duplication and, conversely, overexpression causes centriole amplification and thus disruption of cell division homeostasis (Arquint and Nigg 2016).
Data collected from a large, consanguineous family, identified a homozygous missense mutation (c.185T>C) in the SASS6 gene in patients IV-7, V-1 and V-3 with primary microcephaly (Khan et al. 2014). SASS6 centriolar recruitment is not altered by the Ile62Thr mutation in the patient cells, but the mutant variant cannot sustain centriole formation in cells depleted of endogenous SASS6. Furthermore, DmSas-6 (the Drosophila ortholog of SASS6) knockout larvae exhibited a significantly reduced number of centrosomes in the brain and ∼ 18% of centrosomes were even smaller in size compared with wild-type flies (Ana Rodrigues-Martins et al. 2007a, b). The residual asymmetric cell division resulted in reduced brain development, and, thus, caused primary autosomal recessive microcephaly. This work on the SASS6 mutation reinforces the notion that partial impairment of centriole formation results in MCPH.
Microtubule-associated protein 11 (MAP11/MCPH25)
MAP11, also called C7orf43, is encoded by a gene located at chromosome 7q22.1 and encodes a microtubule-associated protein. By analyzing SH-SY5Y human neuroblastoma cells, Perez et al. found that MAP11 co-localized and physically associated with α-tubulin during mitosis. At the same time, MAP11 is also co-localized with a key regulator of the cytokinesis protein PLK1 at the edges of the microtubule extensions of daughter cells post-cytokinesis abscission, preceding α-tubulin in the formation of the midbody abscission. This finding also suggests that MAP11 is involved in mitotic spindle dynamics and the regulation of cell division. A MAP11 deficiency in zebrafish results in decreased brain cellular proliferation due to a neuronal proliferation deficiency in early zebrafish neurodevelopment; this effect causes a depletion of the progenitor pool that is unable to give rise to a sufficient number of neurons to populate the growing neocortex. Both affected humans and zebrafish mutant lines showed reduced neuronal proliferation and had a smaller head size; it is thus supposed that MAP11 can cause microcephaly in both humans and zebrafish by reducing cell proliferation during early neurodevelopment (Perez et al. 2019).
Kinetochore-associated pathogenic genes
Accurately maintaining chromosome segregation at cell division is essential for cell proliferation and protecting from aneuploidy and chromosomal instability. Kinetochores, centromeres and chromosomes are the central players in chromosome segregation. The kinetochore is a proteinaceous macromolecular structure that forms attachments to the microtubules of the mitotic spindles, and is recruited to the centromere of each chromosome. The correct connections between chromosomes and spindle microtubules are key for error-free chromosome separation. The kinetochore also recruits more proteins to ensure correct attachment of microtubules to the chromosome at SAC. The kinetochore, thus, prevents chromosome separation until all chromosomes are correctly bi-oriented (Stukenberg and Burke 2015; Musacchio 2015). At mitosis, faithful and accurate segregation of the genome also requires chromosome condensation and resolution of sister chromatids to ensure the movement of chromosomes to daughter cells without DNA entanglement or entrapment by the cleavage furrow (Martin et al. 2016). A kinetochore-associated mechanism regulating brain size has been proposed, including MCPH4 and MCPH13.
Kinetochore scaffold 1 (KNL1/MCPH4)
The CASC5/KNL1 gene is located on chromosome 15q and has 27 exons. These exons encode a relatively large, 265-KDa protein localized at the kinetochore. The skipping of exon 18 has been predicted to cause a frameshift mutation and a premature stop codon in exon 19, which would result in either a truncated or absent protein product if subject to nonsense-mediated mRNA decay (Genin et al. 2012; Shi et al. 2017). Homozygosity analysis of two families with primary microcephaly, using genome-wide dense single-nucleotide polymorphism (SNP) genotyping, supported linkage to the published MCPH4 locus on chromosome 15q21.1 (Guernsey et al. 2010). In vertebrates, the kinetochore consists of several sub-complexes, including centromere-associated network (CCAN) and the KMN (KNL1, Mis12, and Ndc80 complexes)-network complexes. The CCAN proteins localize to the centromere throughout the cell cycle, then the KMN proteins, contacting with microtubes, are recruited to the CCAN complex (Hara and Fukagawa 2018). Defects in kinetochore or centromere function can lead to aneuploidy and chromosomal instability, resulting in developmental defects or disease (Fukagawa and Earnshaw 2014).
KNL1 is part of the KMN network, which acts as an interface between the kinetochore and its associated microtubules. It is required for proper microtubule attachment to the centromere and SAC activation during mitosis, and its knockdown is associated with premature entry into mitosis (Genin et al. 2012). The KNL1 p.M2041Ile mutation, detected in an MCPH4 patient, lacks the C terminal domain that specifically interacts with KAT8 regulatory NSL complex subunit 1 (NSL1), ZW10-interacting protein 1 (ZWINT-1) and the Protein MIS12 homolog (MIS12) complex, which function in kinetochore assembly (Kiyomitsu et al. 2011; Petrovic et al. 2010). Correct docking is essential for adequate segregation of the sister chromatids at anaphase and is required for the spindle checkpoint of the mitotic cycle.
A human embryonic stem cell line containing the microcephaly patient-specific KNL1 c.6125G>A mutation, was constructed and displayed impaired proliferation and premature differentiation in neural progenitor cells. These cells rapidly developed an aneuploid karyotype, which may cause microcephaly symptoms (Omer Javed et al. 2018). Recently, a conditional deletion of KNL1 was produced in cortical neural progenitor cells of the embryonic mouse brain. This deletion was associated with a 40% decrease in the cortical area at birth, which is consistent with the human microcephaly phenotype. The segregation errors in mitotic neural progenitor cells, induced by KNL1 depletion, triggered rapid p53 activation and robust apoptotic and microglial phagocytic responses that extensively eliminate cells with somatic genome damage and leave progenitors with a normal karyotype for dividing later, thus causing microcephaly (Shi et al. 2019).
Centromere-associated protein-E (CENPE/MCPH13)
The CENP-E gene, located on chromosome 4q24, encodes for a microtubule plus-end-directed kinesin protein comprised of 2,701 amino acids (Wood et al. 1997). CENP-E promotes spindle microtubule capture and attachment at the kinetochore, and maintains kinetochore–microtubule stability required for chromosome alignment at the equatorial plate in metaphase (Abrieu et al. 2000; Yao et al. 2000; Liu et al. 2007). Once the tail domain of CENP-E is phosphorylated, activated CENP-E changes in conformation to ensure plus-end-directed movements (Espeut et al. 2008). CENP-E transports chromosomes and tethers the kinetochores towards microtubule plus ends. The C-terminal domain of CENP-E interacts with another kinetochore protein Kinetochore protein Nuf2 (NUF2, a component of the essential kinetochore-associated NDC80 complex) and then localizes to the kinetochores. The stable kinetochore–microtubule attachment comprised by CENP-E cooperating with NUF2 is a bridge between spindle microtubules and the kinetochore core complex and regulates chromosome congression. Only 50% of microtubules attached to the kinetochores compared with microtubles in wild-type cells (Putkey et al. 2002). CENP-E is also essential for the spindle assembly checkpoint during mitosis, while chromosome alignment abnormal and mitosis arrest are detected in CENP-E-deficient cells (Rieder et al. 1995). CENP-E directly interacts with mitotic checkpoint serine/threonine-protein kinase BUB1 beta (BubR1) and activates BubR1 kinase to enhance chromosome alignment and the mitotic checkpoint, which prevents aneuploidy (Guo et al. 2012). In humans, compound heterozygous variants in CENP-E (c.2797G>a:p.D933N and c.4063a>G:p.K1355E) have been found in two siblings with microcephaly symptoms (MCPH13) (Mirzaa et al. 2014). The mutation is located in the central coiled-coil region of the CENP-E protein, which is required for the dynamic interaction of CENP-E with microtubules. SAC function and chromosome alignment are also affected (Taveras et al. 2019). Thus, severely decreased CENP-E localization at the centromere and abnormal spindle organization was detected in patient-derived lymphoblastoid cells (Mirzaa et al. 2014). The developing embryonic neuroepithelium is sensitive to perturbations in spindle orientation. As such, alterations in the cleavage plane can disrupt symmetric–asymmetric division, migration and differentiation (Fish et al. 2006). The MCPH13 mutation associated with aberrant mitotic spindle structure, chromosome mis-segregation and delayed mitotic progression represents a significant underlying pathomechanism for microcephalic phenotypes in humans. CENP-E null-induced MCPH might be associated with impaired kinetochore–microtubule stability.
Cytokinesis regulation-associated pathogenic genes
Cell division terminates with cytokinesis and cellular separation. Midbody formation in cytokinesis is the final phase of cell division, and safeguards the execution of the abscission process for the correct distribution of genomic and cytoplasmic materials between the two nascent daughter cells. Visualization of the midbody is essential for delamination, migration, and neuroblast fate (Johnson et al. 2017). A common cytokinesis regulation-related mechanism regulating brain size has been proposed, including MCPH17 and MCPH20 (Li et al. 2016; Fujikura et al. 2013).
Citron Rho-interacting kinase (CIT/MCPH17)
CIT is a 183-kDa, GTP-bound Rac and Rho binding protein that consists of an N-terminal kinase domain, two central coiled-coil domains that include a Rho/Rac-binding domain, and C-terminus domain containing a cysteine-rich motif, a pleckstrin homology domain, and a Citron–Nik1 homology domain (Di Cunto et al. 1998; Madaule et al. 1998).
During anaphase, accumulation of CIT increases substantially at the cleavage furrow, and binds activated Rho and Rac to form the actomyosin contractile ring (Bassi et al. 2013; Watanabe et al. 2013). Constriction of the ring by phosphorylating Myosin regulatory light chain (MRLC) mediated by CIT, leads to furrow ingression and compacts the central spindle to form the midbody (Eda et al. 2001). Furthermore, CIT locates at the center of the intercellular bridge, which acts as a platform linking component of the contractile ring and central spindle for the final separation of the two daughter cells (D'Avino and Capalbo 2016; McKenzie et al. 2016). Loss of CIT leads to the loss of the highly ordered arrangement of midbody proteins and the connection between the contractile ring and central spindle factors, resulting in abscission failure (McKenzie et al. 2016). CIT also promotes Tubulin beta-3 chain (TUBB3) phosphorylation to stabilize microtubules and maintain the structure of the midbody during cell division, and its mutation leads to apoptosis in neural progenitors and newborn neurons (Sgrò et al. 2016; Bianchi et al. 2020).
Besides cytokinesis, CIT accumulates at the spindle poles during metaphase and is also required for the correct orientation of the mitotic spindle during metaphase, where it interacts with ASPM. CIT, thus, has an important role in the organization and polymerization of spindle microtubules throughout cell division (Gai et al. 2016). Spindle microtubule regulation depends on an interaction between CIT and ASPM, and CIT kinase activity. What is more, CIT can mediate the nucleation, length and stability of astral microtubules. The multiple levels of CIT function in regulating cell division suggest its importance in this process.
In Cit−/− mice, affected cytokinesis and increased apoptosis impair the development of the central nervous system. Defective neurogenesis with dramatic depletion of microneurons in the olfactory bulb, hippocampus, and cerebellum is observed (Di Cunto et al. 2000). Lately, three different CIT homozygous missense mutations resulting in null kinase activity were identified in three unrelated consanguineous families with primary microcephaly-17 (Li et al. 2016). The patient-derived induced neural progenitor cells showed a cytokinesis failure with spindles with multiple poles, cell cycle arrest, chromosomal instability, aneuploidy and apoptosis, although no defects in the patient fibroblasts. Thus, a model in at least some forms of microcephaly might be that loss of mitotic integrity through an effect on centrosomes or cytokinesis leads to the instability of genome, resulting in genotoxic stress, apoptosis, and subsequently, a smaller cerebral volume.
CIT also plays a role in DNA damage control (Bianchi et al. 2017). CIT loss compromised RAD51 recruitment at DNA damage sites and chromosomal instability both in mammals and Drosophila. In cortical neural progenitors, CIT loss led to p53-dependent massive apoptosis and neuronal loss throughout brain development. Thus, CIT loss results in a combination of cytokinesis failure, increased DNA damage and chromosomal instability. This also explains why CIT is one of the most severe forms of primary microcephaly (Li et al. 2016).
Kinesin family member 14 (KIF14/MCPH20)
KIF14, which belongs to the kinesin-3 family of microtubule motors, is encoded by a gene located on chromosome 1q32.1, and is comprised of 1,648 amino acids with a molecular weight of 186 kDa. As a mitotic motor protein, KIF14 binds to microtubules with a tubulin heterodimer, and has ATPase activity (Marx et al. 2009; Verhey et al. 2011). KIF14 is a key protein involved in regulating cytokinesis. SiRNA silencing of KIF14 resulted in failed midbody formation and cytokinesis completion, leading to multinucleated cells that later undergo apoptosis (Gruneberg et al. 2006; Carleton et al. 2006). During anaphase, KIF14 localizes at the central spindle via its interaction with protein-regulating cytokinesis 1, which functions as a scaffold for the recruitment of several kinesins to midzones to enable cytokinesis (Gruneberg et al. 2006; Carleton et al. 2006). Importantly, KIF14 and CIT function as co-dependent partners for their localization at the midbody; failed localization of CIT is observed in KIF14-deficient cells, and vice versa, during cytokinesis (Gruneberg et al. 2006). Recently, a KIF14 mutation was identified as the driver of primary microcephaly in four families from Pakistan, Saudi Arabia, and Germany (Moawia et al. 2017). In the patient-derived fibroblasts, KIF14 mRNA levels were lower than controls and neither KIF14 nor CIT appeared at the cytokinesis midbody. Abnormal cytokinesis in these patient-derived fibroblasts was associated with increased numbers of binucleated and apoptotic cells. Homozygous KIF14-mutant mice were also found to have microcephaly and severely disrupted CNS myelination due to increased levels of apoptosis and reduced proliferation during embryogenesis (Fujikura et al. 2013).
Nuclear envelope (NE) regulation-associated pathogenic genes
Eukaryotic chromosomes are enclosed by inner and outer nuclear membranes; here, nuclear pore complexes (NPCs) provide perforations to permit nucleo-cytoplasmic transport (Hetzer et al. 2005). The selective transport of factors into or out of the nucleus via the NPC ensures the accurate progression of most major cellular processes. Additionally, in proliferative cells, NPC proteins are essential for genome integrity maintenance and NE disassembly and reassembly during mitotic progression (Ibarra and Hetzer 2015). A NE regulation-related mechanism regulating brain size has been proposed, including MCPH16 and MCPH24.
Ankyrin repeat and LEM domain-containing protein 2 (ANKLE2/MCPH16)
ANKLE2, also known as LEM4, consists of a hydrophobic LEM domain close to the N-terminus (which might serve as a membrane anchor), two central ankyrin repeats and a C-terminus. ANKLE2 tethers chromatin to the NE and is involved in the control of pre-mitotic NE disassembly and postmitotic NE reassembly (Gay and Foiani 2015). NE disassembly is the first step during mitosis initiation and occurs due to the loss of the chromatin–NE link. This process is mediated by the phosphorylation of barrier-to-autointegration factor (BAF) by serine/threonine-protein kinase VRK1 (VRK1), which binds directly to DNA and ANKLE2 (Asencio et al. 2012). After mitosis, PP2A interacts with ANKLE2 to mediate the dephosphorylation of BAF, which is required for the re-association of this nuclear factor to chromosomes in telophase (Snyers et al. 2018).
The importance of ANKLE2 to normal cell function has been shown by generating CRISPR/Cas9-induced ANKLE2 deficiency in HeLa cells. ANKLE deficiency compromises the post-mitotic re-association of the nuclear proteins BAF, Lamina-associated polypeptide 2, isoform alpha (LAP2α) and LaminA with chromosomes (Snyers et al. 2018). Furthermore, the dynamics of ANKLE2 acetylation at K302 and phosphorylation at S662, regulated by the deacetylase SIRT2, are directly linked to NE reassembly (Kaufmann et al. 2016). ANKLE2 deficiency leads to NE instability during telophase, and can also result in hyperploid cells (Snyers et al. 2018). In two individuals in a family affected by severe microcephaly, a deleterious allele in ANKLE2 was discovered and a severe defect in neuroblast proliferation and excessive apoptosis in the third instar larval brain of dAnkle2 mutants was observed. Whole-exome sequencing linked ANKLE2 variants to microcephaly in humans, and it was determined that ANKLE2 was involved in central nervous system cell development due to rescue lethality and the small brain phenotype seen in the Drosophila mutant dAnkle2 (Yamamoto et al. 2014). It seems that the small brain phenotype in dAnkle2 mutants is caused by a substantial reduction in the number of neuroblasts resulting from defects in proliferation and excessive apoptosis.
Nucleoporin 37 (NUP37/MCPH24)
NUP37 is a 37-kDa protein consisting of 326 amino acids, and is located on chromosome 12q23. It is a component of the Nup107-160 subcomplex of the NPC. The NPC is a supramolecular assembly found at points of fusion between the inner and outer nuclear membranes; it is dispersed along with the disassembly of the nuclear envelope during the prophase–prometaphase transition in metazoans for the further establishment of the mitotic spindle apparatus (Zuccolo et al. 2007). Each NPC is composed of multiple copies of 30 different subunits, known as nucleoporins (Nups). These subunits impact on genome integrity maintenance and mitotic progression and are involved in several DNA metabolism processes such as transcription, chromosome duplication, and segregation (Ibarra and Hetzer 2015).
A homozygous protein-truncation mutation (c.916C>T, p.Arg306*) was found in NUP37 in a consanguineous Pakistani family that included three patients with primary microcephaly. The patient fibroblasts exhibited altered chromatin organization and nucleolar morphology (Braun et al. 2018). In dermal fibroblasts, the NUP37 mRNA and protein levels were reduced, as well as other components of the Nup107-160 complex, such as NUP107. Others showed that a homozygous NUP37 mutation leads to microcephaly with steroid-resistant nephrotic syndrome (Rosti et al. 2017).
After complete disassembly of the NPC, Nup107/160 relocates to the kinetochore, where it might participate in new functions in chromosome congression and normal spindle assembly. Indeed, GFP-tagged NUP37 associates with kinetochores during mitosis (Ibarra and Hetzer 2015; Braun et al. 2018). Silencing NUP37 induces inhibition of cell proliferation, G1-phase cell cycle arrest and apoptosis in non-small cell lung cancer cells. Similarly, NUP37-mutant fibroblasts from an MCPH24 patient also showed a decreased rate of cell proliferation (Huang et al. 2020; Braun et al. 2018).
It, thus, seems that MCPH24 phenotypes might be the result of several cellular perturbations, including chromosome congression defects and abnormal spindle assembly, which are essential for the apical migration of neuronal precursors and the architecture of the developing brain (Degrassi et al. 2019).
Chromosome remodeling- or chromosome condensation-associated pathogenic genes
Chromatin is the physiological template of our genome, possessing highly conserved core histone proteins including H2A, H2B, H3 and H4. The dynamic modulation of chromatin structure by chromatin remodeling is a key mechanism to regulate gene expression, apoptosis, DNA replication and repair, and chromosome condensation and segregation (Wang et al. 2007). Recent studies shed light on the importance of chromatin regulatory complexes, including the polycomb and trithorax group complex (PcG and TrxG), in brain development and developmental disorders (Ma et al. 2010). PcG-mediated histone modifications and DNA methylation are required for neural differentiation over many cell divisions to promote self-renewal of adult neural stem cells in the subventricular zone. The TrxG complex promotes switching neural stem cells from a PcG-mediated self-renewal state into a neuronal differentiation state by coupling with PcG antagonists (Ma et al. 2010). Chromosome condensation is also essential for embryonic stem cell differentiation into neural progenitor cells, while the chromatin state is globally open in embryonic stem cells. Of note, chromatin condensation on a large scale during neocortical development would lead to a loss of neurogenic potential (Kishi and Gotoh 2018). A chromosome remodeling- or chromosome condensation-related mechanism regulating brain size was proposed, including MCPH10, MCPH11 and MCPH21-23.
Zinc finger protein 335 (ZNF335/MCPH10)
ZNF335, also known as nuclear hormone receptor transcriptional coactivator (NRC)-interacting factor 1 (NIF-1), is a coregulator of nuclear hormone signaling. ZNF335 is implicated in chromatin remodeling and is a novel component of a vertebrate-specific TrxG complex for H3K4 methylation that works with PcG in opposition (Ng and Gurdon 2008). The TrxG complex activates genes through H3K4 methylation. Gene targets include those involved in patterning, cell proliferation and stem cell identity (Fisher and Fisher 2011; Papp and Müller 2006).
A hypomorphic allele of the ZNF335 gene was sequenced and a splice donor/missense mutation (R1111H) was detected in affected members of an Arab Israeli family with MCPH10 (Yang et al. 2012). Functional analysis of this pathogenic variant in patient cells revealed reduced ZNF335 expression with decreased mRNA levels of RE1-silencing transcription factor (REST) and H3K4me3 marks at the REST promoter. REST is expressed in neural stem cells and is essential for maintaining progenitor cell fate by inhibiting neuronal-specific genes. The study identified that ZNF335, as an essential link between H3K4 complexes and REST, is involved in the pathway that regulates human neurogenesis and neuronal differentiation (Sun et al. 2005). Furthermore, ZNF335 can indirectly influence the functions of various transcription factors, such as NF-κB and c-Jun (Garapaty et al. 2009). It also binds to the transcriptional coregulator HCFC1, which has chromatin remodeling activity (Yang et al. 2012; Trynka et al. 2011). Downregulation of ZNF335 in both E9.5 and E12.5 neuroprogenitors results in a decrease in reaggregated sphere formation, which disrupts progenitor cell proliferation and neuronal differentiation (Barbelanne and Tsang 2014; Yang et al. 2012). Similarly, Znf335 conditional knockout in mice leads to severely reduced cortical size, while Znf335-null mice are embryonically lethal (Yang et al. 2012). Finally, ZNF335 is important for both normal human brain development and early embryonic mouse development. Thus, ZNF335 might form part of a new pathway that coordinates global transcriptional regulation during brain development to affect cell fate and cause microcephaly.
Polyhomeotic-like protein 1 (PHC1/MCPH11)
The PHC1 gene is located on chromosome 12p13.31, and encodes PHC1 protein that is a core component of the canonical Polycomb repressive complex 1 (PRC1). PHC1 is a human homolog component of a polycomb group multiprotein PRC1-like complex in Drosophila. PRC1 is an inhibitor of initial transcription: it prevents ATP-dependent nucleosome remodeling and acts as an E3 ubiquitin ligase of H2A mono-ubiquitination (H2AUb1) (Wang et al. 2004). H2AUb1, a chromatin modification, has an important role in the epigenetic establishment of mature cell fates by protecting CpG islands against DNA methylation; it acts as a stable and heritable repressive epigenetic mark essential for development and for generating a diversity of cell fates from multipotent progenitor cells (Cao et al. 2005; Boulard et al. 2015; Wu et al. 2013). A deficiency in neuronal differentiation, caused by germline pathogenic variants in components of the H2AUb1 regulatory axis, has been identified as the genetic basis of congenital neurodevelopmental disorders (Cao et al. 2005; Sparmann and Van Lohuizen 2006).
A novel missense PHC1 variant in a consanguineous family with primary microcephaly was identified as a genetic basis for MCPH11 (OMIM; 615414). This finding links primary microcephaly to abnormal chromatin modifications for the first time (Awad et al. 2013). The missense mutation in the identified patient cells impairs the domain of PHC1 that forms a complex with H2A and promotes the proteasome-mediated degradation of PHC1. These defects are accompanied by the increased expression of Geminin and reduced proliferative capacity (L. Luo and Kessel 2004). The pathogenesis of primary microcephaly could be due in part to dysregulated H2AUb1 modification exchange or to cell cycle abnormalities as a consequence of geminin accumulation.
Non-SMC condensing I complex subunit D2 (NCAPD2/MCPH21) Non-SMC condensing I complex subunit D3 (NCAPD3/MCPH22), and NCAPH (MCPH23)
Human NCAPD2 is found on chromosome 12p13.31, and NCAPH is located on chromosome 2q11.2. NCAPD2 and NCAPH are two of the three associated non-SMC subunits of the condensin I complex. NCAPD3 is found on chromosome 11q25, and is one of the three associated non-SMC subunits of the condensin II complex (Hirano et al. 1997). The condensin I and II complexes share the same structural maintenance mechanisms as chromosome ATPase subunits (SMC2 and SMC4), and have a central role in mitotic chromosome assembly and segregation (Hirano 2016; Wood et al. 2010). Timely condensation of the chromosomes is necessary for accurate segregation of the genome during mitosis. Condensin II is localized in the nucleus throughout the cell cycle and helps to sort early axial of chromosomes in prophase. Meanwhile, condensin I shifts from the cytoplasm into the nucleus, and contributes to the lateral compaction of chromosomes during metaphase (Hirota et al. 2004; Shintomi and Hirano 2011). Condensins protect chromosomes moving to the daughter cells from DNA entanglement by facilitating topoisomerase II to actively resolve entangled sister chromatids or entrapment by the cleavage furrow (Hirano 2012; Charbin et al. 2014). In apical progenitors from mice, a condensin II mutation (Ncaph2I15N/I15N) was found together with frequent chromatin bridges during anaphase with unaltered spindle orientation, leading to chromosome segregation errors and impaired cell survival (Martin et al. 2016).
Recently, genomic DNA from patients with microcephaly was sequenced and a splice site mutation (c.4120+2T>C) in NCAPD2, a missense mutation (c.3458T>G) in NCAPD3, a frameshift mutation (c.1783_1784delG) and an intronic substitution (c.382+14A>G) in NCAPD3 and a missense mutation (c.728C>T) in NCAPH were found. In fibroblasts with NCAPD2 or NCAPD3 mutations, elevated levels of DNA bridges confirmed that such mitotic abnormalities also occurred in condensin microcephaly patients. Chromosome segregation errors and subsequent aneuploidy/micronucleus formation induced by decatenation failure ultimately lead to a reduced brain size due to a reduction in the number of cells generated during neurogenesis: the end result is microcephaly.
Notably, the protein encoded by the primary microcephaly MCPH1 gene is a regulator of chromosome condensation, and inhibits condensin II through its interaction domain that includes residues 381–435 in exon 8. This domain interacts with both NCAPD3 and NCAPG2 subunits (Trimborn et al. 2006; Yamashita et al. 2011). The identification of early cell cycle progression and premature chromosome condensation in MCPH1 patients raises the possibility that similar mechanisms that underlie decatenation failure, caused by a loss of MCPH1, could contribute to a reduced brain size.
Mitosis machinery-irrelevant pathogenic genes
Genetic causes of microcephaly continue to grow in diversity and include proteins involved in membrane transport, autophagosomes and vesicle trafficking. It seems that not only the mitosis machinery (including the centrioles, spindles or chromatin proteins) cause microcephaly, but also other cell development pathway-organized proteins can result in premature neuronal fate specification, with a consequent loss of progenitor cells. A mitosis machinery-irrelevant mechanism regulating brain size has been proposed, including MCPH15, MCPH18 and MCPH19.
Major facilitator superfamily domain containing 2a (MFSD2A/MCPH15)
MFSD2A, which is located on chromosome 1p34.2, encodes a 60-kDa protein that functions as membrane transport protein during blood–brain barrier formation and function (Di Cunto et al. 1998; Madaule et al. 1998). The blood–brain barrier, localized at the level of tight junctions between adjacent brain endothelial cells, ensures that metabolites and essential nutrients reach growing brains and prevents the buildup of toxic plasma and cell constituents. MFSD2A is a sodium-dependent transporter that transports docosahexaenoic acid (DHA) (Andreone et al. 2017; Chan et al. 2018). DHA is not efficiently synthesized in the brain; thus, MFSD2A facilitates the transport of DHA across the blood–brain barrier, esterified as lysophosphatidylcholine-DHA (LPC-DHA). Decreased LPC-DHA levels in the developing brain have a negative impact on cognitive function (Guesnet and Alessandri 2011; McNamara 2010), and thus have been implicated in various neurodevelopmental disorders (Colombo et al. 2004; Heird and Lapillonne 2005; Martinez 1996).
Three separate families with homozygous nonsynonymous loss-of-function mutations in MFSD2A have presented with severe microcephaly and intellectual disability, further highlighting the association between MFSD2A and this condition (Guemez-Gamboa et al. 2015; Alakbarzade et al. 2015). The identified MFSD2A mutations were all localized to the transmembrane domains of the protein. In the affected individuals, plasma concentrations of LPCs containing mono- and polyunsaturated fatty acyl chains were significantly increased. As such, these patients exhibited low LPC uptake in the brain (Alakbarzade et al. 2015). Defects in MFSD2A can, therefore, result in decreased DHA levels in the brain, which leads to a loss of neurons in the hippocampus and cerebellum, cognitive deficits, severe anxiety and microcephaly (Guemez-Gamboa et al. 2015).
Autophagy-linked FYVE (WDFY3/MCPH18)
The WDFY3 gene, also known as ALFY, is located on chromosome 8p23 and is strongly expressed in the developing brain and spleen. Here, it acts as a master scaffold protein for autophagy (Simonsen et al. 2004). In the cytoplasm, ALFY is recruited to intracellular inclusions and links p62-associated ubiquitinated proteins to core autophagic effectors, including ATG5–ATG12–ATG16L E3-like ligase and LC3, and forms autophagosomes for protein disintegrated (Filimonenko et al. 2010). Mediated by autophagy, Wnt signaling could be negatively regulated by promoting the degradation of disheveled (DVL) proteins, which relay Wnt signals from receptors to downstream effectors (Gao and Chen 2010). The ALFY protein is highly expressed in the developing CNS and crucial for the development of axonal tracts (Naveed et al. 2018).
A missense mutation in ALFY (c.7909C>T) was identified in an affected Arab Israeli family with congenital microcephaly accompanied with symptoms of mild-to-moderate intellectual disability (Kadir et al. 2016). The mutation causes an R2637W substitution at a highly conserved residue in the PH-BEACH domain, which is the key domain for ALFY binding to p62 (Clausen et al. 2010). The ALFY mutation identified in the affected patients abrogates the normal function of ALFY to attenuate Wnt signaling, which is one of the major molecular pathways controlling cell division and the transition between symmetrical and non-symmetrical cell division during normal brain development (Kuwahara et al. 2010; Bengoa-Vergniory et al. 2014).
Further research found that ALFY was involved in the DVL autophagy pathway that regulates Wnt signaling: Drosophila expressing the ALFY mutation exhibited primary microcephaly and the affected patients also had smaller brains (Kadir et al. 2016). Increased levels of segment polarity protein disheveled homolog DVL-3 (DVL3) cause abnormal Wnt signaling activation. It is thought that ALFY-mediated abnormal Wnt signaling activation induces the continued generation of apical progenitor cells, which then fail to differentiate and form the basal progenitor cell layers in the cerebral cortex (Kadir et al. 2016).
Coatomer subunit β (COPB2/MCPH19)
The COPB2 gene is located on chromosome 3q23 and encodes the β-COP protein. β-COP is comprised of 906 amino acids with a molecular weight ∼102 kDa, and is a subunit of the Golgi Coatomer complex. β-COP is necessary for retrograde trafficking from the Golgi apparatus to the endoplasmic reticulum, interacting both with the cargo and with regulators of intracellular trafficking (Letourneur et al. 1994; Styers et al. 2008; Waters et al. 1991). The critical importance of the Golgi apparatus (GA) for neural function has been reported and > 40% of GA-related genes are associated with central or peripheral nervous system diseases. It has been proposed that neocortical development is sensitive to ER/Golgi stress due to polarity establishment and maintenance (which occurs at every step of corticogenesis), both are highly dependent on traffic through the secretory pathway (Rasika et al. 2019).
Recently, a homozygous missense mutation in the COPB2 gene (R254C) was identified in two siblings with primary microcephaly-19 (DiStasio et al. 2017). Genome-editing technology was subsequently used to generate a homozygous/heterozygous allele carrying the patient variant in the mouse to explore the role of Copb2 in neural development. Compared with mice homozygous for the patient variant (Copb2R254C/R254C), compound heterozygous mutants for a null Copb2 allele and R254C showed a severe perinatal phenotype and reduced amount of cortical tissue. Immunostaining of the Copb2R254C/Zfn brain revealed significantly increased levels of apoptosis and a reduction in layer V (CTIP2+) neurons. Considering the decreased level of proliferation in neurospheres derived from mutant animal is associated with a defect in ventricular zone (VZ) proliferation and layer V proliferation, it seems that primary microcephaly-19 is caused by a discrepancy in the ratio of symmetrical to asymmetrical divisions among progenitor cells (DiStasio et al. 2017).
Primary microcephaly and DNA replication
DNA replication is constantly challenged by various endogenous and environmental agents, which can trigger the DNA replication checkpoint. Proper DNA replication and a DNA replication stress response ensures the integrity of the replication process and that replication only occurs once per cell cycle, ultimately preventing genome instability. In our pioneering work, we discovered the novel function of MCPH1 in the DNA damage response. Specifically, we showed that MCPH1 can be involved in the regulation of the intra-S-phase checkpoint (Xu et al. 2004). The public protein–protein interaction database BioGRID shows that MCPH1 potentially interacts with MCM2/5, major components of the cell division control protein 45 homolog (CDC45)/MCM2–7/DNA replication complex Go-Ichi-Ni-San (GINS) (CMG) helicase driving the DNA replication, and TopBP1 that is an activator of the replication checkpoint kinase ATR (https://thebiogrid.org/122776). A recent proteome-wide identification of proteins on the replication fork by the iPOND (isolation of proteins on nascent DNA) methodology uncovered that multiple MCPH proteins including MCPH1, ASPM, CIT, COPB2 and NCAPD2 resided on the replication fork (Dungrawala et al. 2015). These observations imply a potential functional link between MCPH gene function and DNA replication and/or DNA replication stress response. Studies are, thus, warranted to explore if a defect in DNA replication and/or the DNA replication stress response in neuroprogenitor cells contributes to microcephaly in MCPH patients.
Primary microcephaly and tumorigenesis
As we have highlighted, most MCPH genes encode proteins involved in cell cycle regulation, cell division, DNA repair, and the assembly and regulation of the mitosis machinery including centrosomes, microtubules and chromosomes, contributing to the correct distribution of genomic DNA to daughter cells and the stability of genome. Genome instability is a key feature of various human cancers. Data collected from the Cancer Genome Atlas Programs (TCGA) revealed the widespread alterations (mutations, amplifications, and deletions) in MCPH genes in various cancers (Table 2), strongly arguing that MCPH genes modulate tumorigenesis and might, thus, constitute therapeutic targets. Some MCPH genes function to suppress tumor growth and proliferation. Downregulated MCPH1 has been reported in many malignant tumors, including breast cancer, lung cancer, ovarian cancer, oral squamous cell carcinoma and prostate cancer (Mai et al. 2014; Rai et al. 2006; Venkatesh et al. 2013; Wu et al. 2018). MFSD2A is a novel lung cancer tumor suppressor gene that upon downregulation, leads to the G1 arrest in non-small cell lung cancer cell lines and primary lung adenocarcinomas (Spinola et al. 2010). While other MCPH genes are highly expressed in multiple cancers (Table 3). The inactivation of most MCPH genes leads to neurogenesis defects, while upregulation of some MCPH genes resulted in carcinogenesis (Zhou et al. 2020).
Table 2 Alterations in MCPH genes in human cancers
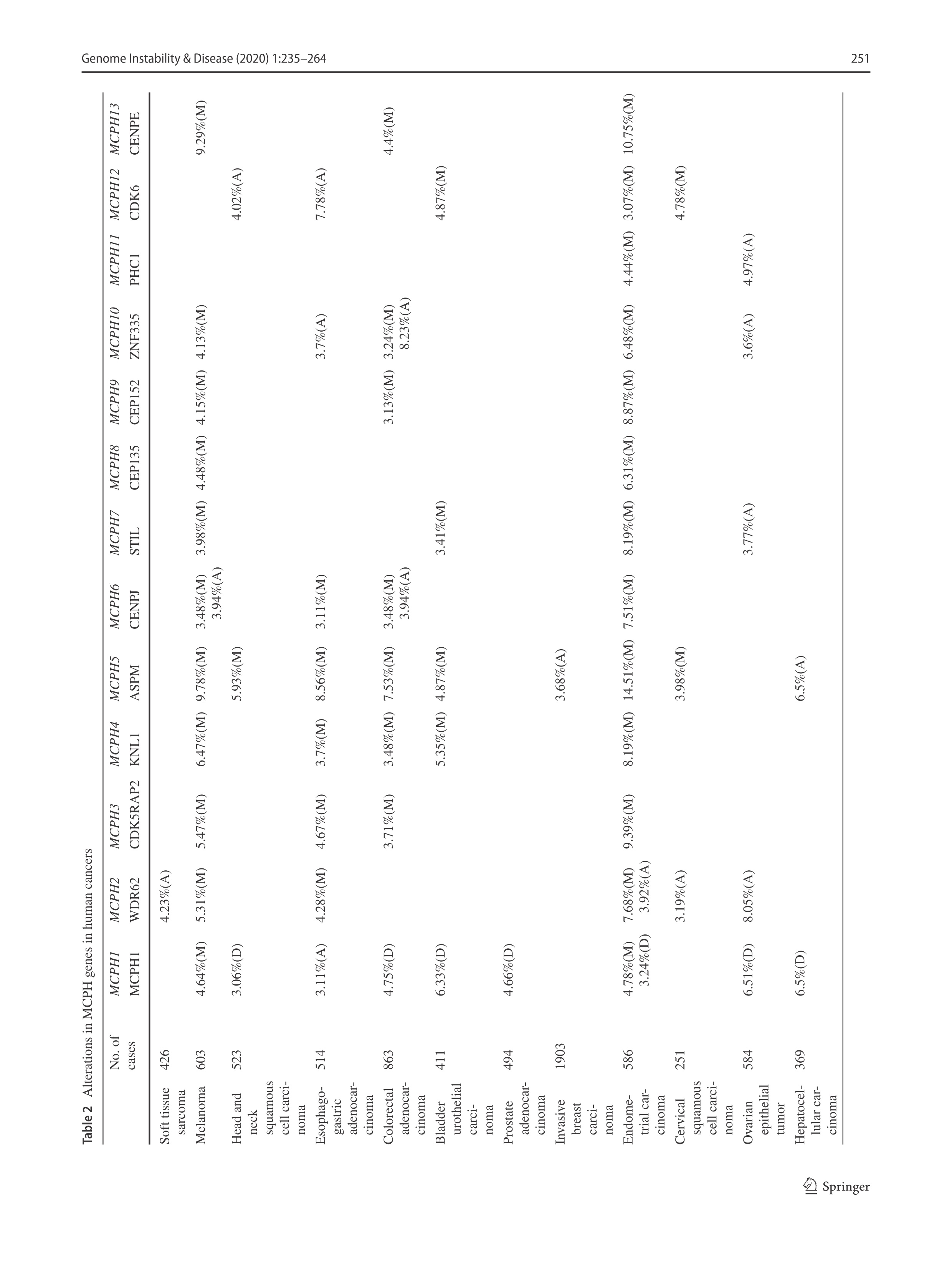
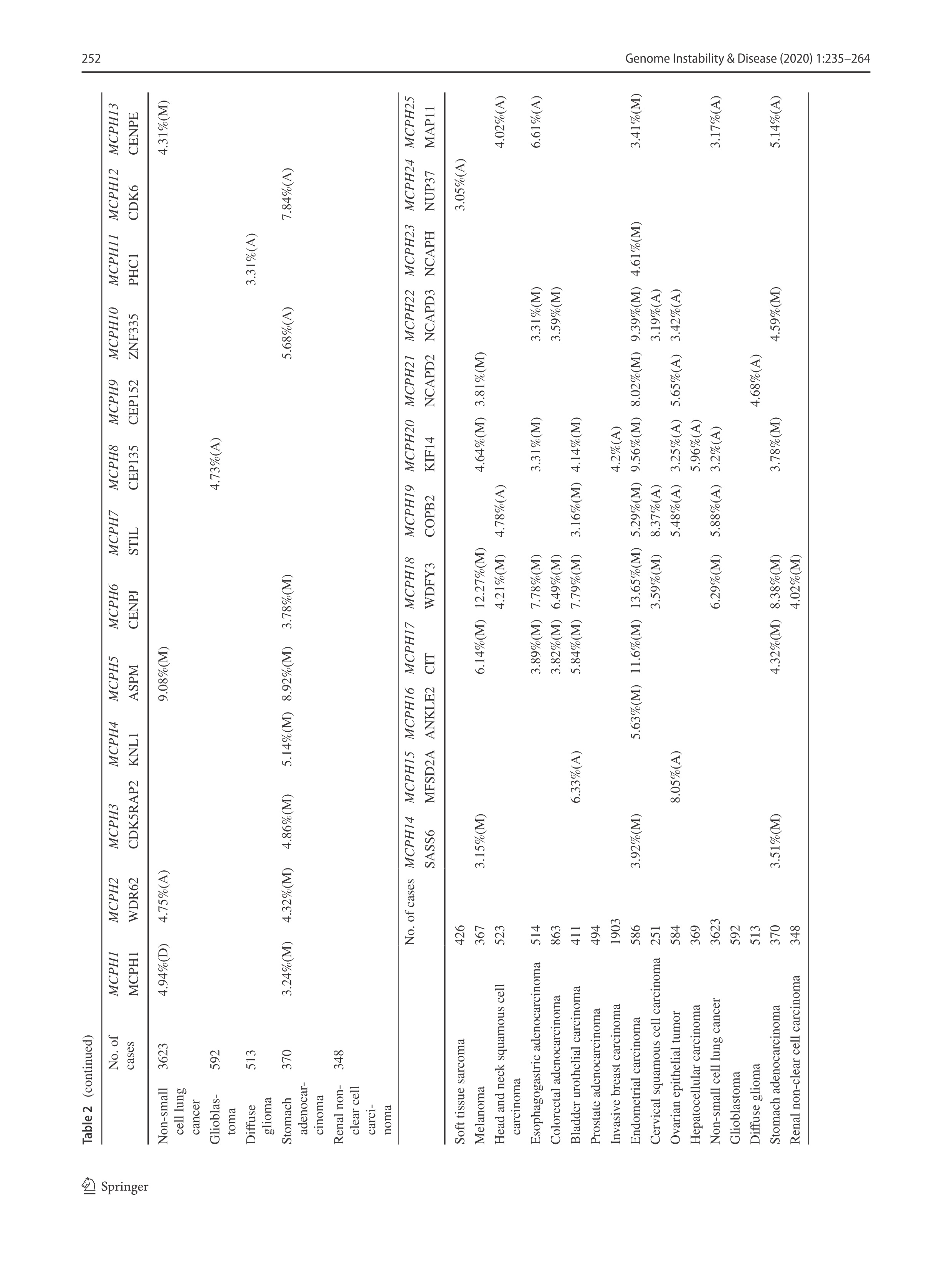
Table 3 MCPH proteins regulate carcinogenesis, tumor prognosis and/or invasion
The coordinated regulation of stem cell self-renewal and differentiation is essential for both the development of the nervous system and the cancer relapse and metastasis by cancer stem cells (CSCs) (Azzarelli et al. 2018). CSCs with a tumor initiating potential actively employ several signal cascades including the Wnt pathway, the Sonic hedgehog (shh) pathway, the Hippo pathway and the Notch pathway, which are utilized by several MCPH genes as well. Through Wnt signal pathway, ASPM augments Wnt–Dvl-3–β-catenin signaling, promoting prostate cancer stemness and progression, while ASPM deficiency leads to the abnormal proliferation and differentiation of neuroprogenitors that could be rescued by co-expression of stabilized β-catenin (Pai et al. 2019; Buchman et al. 2011). CDK6 can activate the Shh signaling to control the onset of cortical neurogenesis, while the Shh signaling can increase MCPH12 expression to promote uncontrolled cell proliferation in medulloblastoma (Raleigh et al. 2018; Hasenpusch-Theil et al. 2018). The Notch pathway modulated by CDK6 (Jena et al. 2016) and the Hippo pathway modulated by CDK5RAP2, COPB2, KIF14 and NUP37 (Sukumaran et al. 2017; Pu et al. 2018; Zhang et al. 2017; Luo et al. 2017), when mutated, disrupt cell cycle regulation and the apoptotic pathway, further suggesting that the similar mechanism is being shared in neurogenesis and carcinogenesis (Zhou et al. 2020). Together, MCPH genes as mentioned above involving stem cell self-renewal by the Wnt, Shh, Notch and Hippo pathways are closely interlinked with tumorigenesis, and these cancer stem cell pathways are potential targets for cancer therapy.Centrosomes are essential for chromosomal stability, and abnormalities of their number or structure affect cell division. Errors in chromosome segregation caused by centrosome amplification and multipolar spindles-induced aneuploidy have been defined as a common feature of cancer cells (Fukasawa 2005). Similar to primary microcephaly, the existence of an important connection between centrosome abnormalities and cancer has been linked by MCPH proteins that regulate the integrity of centrosomes. That means neurogenesis and carcinogenesis may result from similar centriole biogenesis. Centrosome amplification has been observed in ovarian cancer cells with WDR62 overexpression (Zhang et al. 2013a, b). STIL is upregulated in several cancers that confer a poor prognosis, including lung cancer, colon carcinoma, prostate adenocarcinoma and ovarian cancer (Patwardhan et al. 2018). SASS6 overexpression has been linked to mitotic chromosomal abnormalities and a poor prognosis in colorectal cancer patients (Shinmura et al. 2015). Furthermore, CENPE, STIL, CEP135, CEP152 and SASS6, all of which are important regulators of the centrosome duplication process, are also aberrantly expressed in melanoma and esophagogastric adenocarcinoma tumorigenesis (Table 2). Additionally, in a pathway independent of chromosome instability, increasing centrosome amplification has a close relationship with the evolution of cancer cells from a benign tumor to an invasive metastasis, a hallmark of aggressive cancers (Nigg et al. 2014). The overexpression of WDR62, ASPM, STIL, CEP135 and SASS6 is also associated with aggressiveness and poor outcomes in multiple cancers, while these MCPH proteins functions in centriole biogenesis, a common defect underlying genetic microcephaly (Table 3). These data strengthen previous findings that centrosome aberrations and the clinical aggressiveness of tumors are closely interlinked (Nigg et al. 2014).
Concluding remarks
In summary, we have discussed MCPH gene function in centriole biogenesis, mitotic spindle assembly, the DNA damage response, tumorigenesis, and potentially DNA replication and the DNA replication stress response. A better understanding of this rare neuropediatric disorder will not only shed light on brain development, but also uncover new regulatory networks of tumorigenesis and create new strategies for potential targeted therapies.
Abbreviations
BAF:
Barrier-to-autointegration factor
BRCT:
Breast cancer carboxyl terminal domains
CCAN:
Centromere-Associated Network
CMG:
Cell division control protein 45 homolog (CDC45)/MCM2–7/DNA replication complex Go-Ichi-Ni-San (GINS)
CNS:
Central nervous system
CSCs:
Cancer stem cells
DHA:
Docosahexaenoic acid
LPC-DHA:
Lysophosphatidylcholine-DHA
MCM:
Mini-chromosome maintenance (MCM) complex
MCPH:
Autosomal primary microcephaly
MTOC:
Microtubule organizing centers
NE:
Nuclear envelope
NESCs:
Neuroepithelial stem cells
NPCs:
Nuclear pore complexes
NRC:
Nuclear hormone receptor transcriptional coactivator
Nups:
Nucleoporins
PCM:
Pericentriolar material
PcG:
The polycomb group complex
PRC1:
Protein-regulating cytokinesis 1
RA:
Retinoic acid
RB:
Retinoblastoma-associated protein
SAC:
Spindle-assembly checkpoint
SCL/TAL1:
T-cell acute lymphocytic leukemia protein 1(TAL1)
TrxG:
The trithorax group complex
References
Abdel-Hamid, M. S., Ismail, M. F., Darwish, H. A., Effat, L. K., Zaki, M. S., & Abdel-Salam, G. M. H. H. (2016). Molecular and phenotypic spectrum of ASPM-related primary microcephaly: Identification of eight novel mutations. American Journal of Medical Genetics, Part A, 170, 2133–2140. https://doi.org/10.1002/ajmg.a.37724.
Abrieu, A., Kahana, J. A., Wood, K. W., & Cleveland, D. W. (2000). CENP-E as an essential component of the mitotic checkpoint in vitro. Cell. https://doi.org/10.1016/S0092-8674(00)00070-2.
Akbariazar, E., Ebrahimpour, M., Akbari, S., Arzhanghi, S., Abedini, S. S., Najmabadi, H., et al. (2013). A novel deletion mutation in ASPM gene in an Iranian family with autosomal recessive primary microcephaly. Iranian Journal of Child Neurology. https://doi.org/10.22037/ijcn.v7i2.3840.
Akhmanova, A., & Steinmetz, M. O. (2008). Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nature Reviews Molecular Cell Biology, 9, 309–322.
Alakbarzade, V., Hameed, A., Quek, D. Q. Y., Chioza, B. A., Baple, E. L., Cazenave-Gassiot, A., et al. (2015). A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nature Genetics. https://doi.org/10.1038/ng.3313.
Alieva, I. B., & Uzbekov, R. E. (2008). The centrosome is a polyfunctional multiprotein cell complex. Biochemistry (Moscow), 73(6), 626–643. https://doi.org/10.1134/S0006297908060023.
Amisaki, M., Tsuchiya, H., Sakabe, T., Fujiwara, Y., & Shiota, G. (2019). Identification of genes involved in the regulation of TERT in hepatocellular carcinoma. Cancer Science, 110(2), 550–560. https://doi.org/10.1111/cas.13884.
An, C., Li, H., Zhang, X., Wang, J., Qiang, Y., Ye, X., et al. (2019). Silencing of COPB2 inhibits the proliferation of gastric cancer cells and induces apoptosis via suppression of the RTK signaling pathway. International Journal of Oncology, 54, 1195–1208. https://doi.org/10.3892/ijo.2019.4717.
Andreone, B. J., Chow, B. W., Tata, A., Lacoste, B., Ben-Zvi, A., Bullock, K., et al. (2017). Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. https://doi.org/10.1016/j.neuron.2017.03.043.
Arquint, C., & Nigg, E. A. (2016). The PLK4-STIL-SAS-6 module at the core of centriole duplication. Biochemical Society Transactions, 44, 1253–1263.
Asencio, C., Davidson, I. F., Santarella-Mellwig, R., Ly-Hartig, T. B. N., Mall, M., Wallenfang, M. R., et al. (2012). Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell, 150, 122–135. https://doi.org/10.1016/j.cell.2012.04.043.
Awad, S., Al-Dosari, M. S., Al-Yacoub, N., Colak, D., Salih, M. A., Alkuraya, F. S., et al. (2013). Mutation in PHC1 implicates chromatin remodeling in primary microcephaly pathogenesis. Human Molecular Genetics, 22, 2200–2213. https://doi.org/10.1093/hmg/ddt072.
Azzarelli, R., Simons, B. D., & Philpott, A. (2018). The developmental origin of brain tumours: a cellular and molecular framework. Development. https://doi.org/10.1242/dev.162693.
Bai, T., Zhao, Y., Liu, Y., Cai, B., Dong, N., & Li, B. (2019). Effect of KNL1 on the proliferation and apoptosis of colorectal cancer cells. Technology in Cancer Research & Treatment. https://doi.org/10.1177/1533033819858668.
Barbelanne, M., & Tsang, W. Y. (2014). Molecular and cellular basis of autosomal recessive primary microcephaly. BioMed Research International.
Barr, A. R., Kilmartin, J. V., & Gergely, F. (2010). CDK5RAP2 functions in centrosome to spindle pole attachment and DNA damage response. Journal of Cell Biology. https://doi.org/10.1083/jcb.200912163.
Bassi, Z. I., Audusseau, M., Giovanna, M., Callaini, G., Paolo, P., Avino, D., et al. (2013). Citron kinase controls a molecular network required for midbody formation in cytokinesis. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1301328110.
Basto, R., Lau, J., Vinogradova, T., Gardiol, A., Woods, C. G., Khodjakov, A., et al. (2006). Flies without centrioles. Cell, 125, 1375–1386. https://doi.org/10.1016/j.cell.2006.05.025.
Bazzi, H., & Anderson, K. V. (2014). Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo. Proceedings of the National Academy of Sciences of the United States of America, 111(15), E1491–1500. https://doi.org/10.1073/pnas.1400568111.
Bengoa-Vergniory, N., Gorroño-Etxebarria, I., González-Salazar, I., & Kypta, R. M. (2014). A switch from canonical to noncanonical wnt signaling mediates early differentiation of human neural stem cells. Stem Cells. https://doi.org/10.1002/stem.1807.
Beukelaers, P., Vandenbosch, R., Caron, N., Nguyen, L., Belachew, S., Moonen, G., et al. (2011). Cdk6-dependent regulation of G1 length controls adult neurogenesis. Stem Cells, 29, 713–724. https://doi.org/10.1002/stem.616.
Bianchi, F. T., Gai, M., Berto, G. E., & Di Cunto, F. (2020). Of rings and spines: The multiple facets of Citron proteins in neural development. Small GTPases, 11(2), 122–130. https://doi.org/10.1080/21541248.2017.1374325.
Bianchi, F. T., Tocco, C., Pallavicini, G., Liu, Y., Vernì, F., Merigliano, C., et al. (2017). Citron kinase deficiency leads to chromosomal instability and TP53-sensitive microcephaly. Cell Reports, 18, 1674–1686. https://doi.org/10.1016/j.celrep.2017.01.054.
Bilgüvar, K., Öztürk, A. K., Louvi, A., Kwan, K. Y., Choi, M., Tatli, B., et al. (2010). Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. https://doi.org/10.1038/nature09327.
Blachon, S., Gopalakrishnan, J., Omori, Y., Polyanovsky, A., Church, A., Nicastro, D., et al. (2008). Drosophila asterless and vertebrate Cep152 are orthologs essential for centriole duplication. Genetics. https://doi.org/10.1534/genetics.108.095141.
Bogoyevitch, M. A., Yeap, Y. Y. C., Qu, Z., Ngoei, K. R., Yip, Y. Y., Zhao, T. T., et al. (2012). WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression. Journal of Cell Science. https://doi.org/10.1242/jcs.107326.
Bond, J., Roberts, E., Mochida, G. H., Hampshire, D. J., Scott, S., Askham, J. M., et al. (2002). ASPM is a major determinant of cerebral cortical size. Nature Genetics, 32, 316–320. https://doi.org/10.1038/ng995.
Bond, J., Roberts, E., Springell, K., Lizarraga, S. B., Scott, S., Higgins, J., et al. (2005). A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nature Genetics, 37(4), 353–355. https://doi.org/10.1038/ng1539.
Bond, J., & Woods, C. G. (2006). Cytoskeletal genes regulating brain size. Current Opinion in Cell Biology, 18(1), 95–101. https://doi.org/10.1016/j.ceb.2005.11.004.
Boulard, M., Edwards, J. R., & Bestor, T. H. (2015). FBXL10 protects Polycomb-bound genes from hypermethylation. Nature Genetics. https://doi.org/10.1038/ng.3272.
Braun, D. A., Lovric, S., Schapiro, D., Schneider, R., Marquez, J., Asif, M., et al. (2018). Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. Journal of Clinical Investigation, 128, 4313–4328. https://doi.org/10.1172/JCI98688.
Brown, N. J., Marjanović, M., Lüders, J., Stracker, T. H., & Costanzo, V. (2013). Cep63 and Cep152 cooperate to ensure centriole duplication. PLoS ONE. https://doi.org/10.1371/journal.pone.0069986.
Brüning-Richardson, A., Bond, J., Alsiary, R., Richardson, J., Cairns, D. A., McCormack, L., et al. (2011). ASPM and microcephalin expression in epithelial ovarian cancer correlates with tumour grade and survival. British Journal of Cancer, 104, 1602–1610. https://doi.org/10.1038/bjc.2011.117.
Buchman, J. J., Durak, O., & Tsai, L. H. (2011). ASPM regulates Wnt signaling pathway activity in the developing brain. Genes and Development, 25(18), 1909–1914. https://doi.org/10.1101/gad.16830211.
Calegari, F., & Huttner, W. B. (2003). An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. Journal of Cell Science, 116, 4947–4955. https://doi.org/10.1242/jcs.00825.
Cao, R., Tsukada, Y. I., & Zhang, Y. (2005). Role of Bmi-1 and Ring1A in H2A ubiquitylation and hox gene silencing. Molecular Cell. https://doi.org/10.1016/j.molcel.2005.12.002.
Carleton, M., Mao, M., Biery, M., Warrener, P., Kim, S., Buser, C., et al. (2006). RNA interference-mediated silencing of mitotic kinesin KIF14 disrupts cell cycle progression and induces cytokinesis failure. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.26.10.3853-3863.2006.
Chan, J. P., Wong, B. H., Chin, C. F., Galam, D. L. A., Foo, J. C., Wong, L. C., et al. (2018). The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biology. https://doi.org/10.1371/journal.pbio.2006443.
Chandrashekar, D. S., Bashel, B., Balasubramanya, S. A. H., Creighton, C. J., Ponce-Rodriguez, I., Chakravarthi, B. V. S. K., et al. (2017). UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia, 19(8), 649–658. https://doi.org/10.1016/j.neo.2017.05.002.
Chang, C. W., Hsu, W. B., Tsai, J. J., Tang, C. J. C., & Tang, T. K. (2016). CEP295 interacts with microtubules and is required for centriole elongation. Journal of Cell Science. https://doi.org/10.1242/jcs.186338.
Charbin, A., Bouchoux, C., & Uhlmann, F. (2014). Condensin aids sister chromatid decatenation by topoisomerase II. Nucleic Acids Research. https://doi.org/10.1093/nar/gkt882.
Cheffer, A., Tarnok, A., & Ulrich, H. (2013). Cell cycle regulation during neurogenesis in the embryonic and adult brain. Stem Cell Reviews and Reports, 9(6), 794–805. https://doi.org/10.1007/s12015-013-9460-5.
Chen, J., Ingham, N., Clare, S., Raisen, C., Vancollie, V. E., Ismail, O., et al. (2013). Mcph1-deficient mice reveal a role for MCPH1 in otitis media. PLoS ONE, 8(3), e58156. https://doi.org/10.1371/journal.pone.0058156.
Cherkaoui Jaouad, I., Zrhidri, A., Jdioui, W., Lyahyai, J., Raymond, L., Egéa, G., et al. (2018). A novel non sense mutation in WDR62 causes autosomal recessive primary microcephaly: A case report. BMC Medical Genetics. https://doi.org/10.1186/s12881-018-0625-6.
Chou, E. J., Hung, L. Y., Tang, C. J. C., Hsu, W. B., Wu, H. Y., Liao, P. C., et al. (2016). Phosphorylation of CPAP by aurora-A maintains spindle pole integrity during mitosis. Cell Reports, 14, 2975–2987. https://doi.org/10.1016/j.celrep.2016.02.085.
Choudhury, R., Bonacci, T., Arceci, A., Lahiri, D., Mills, C. A., Kernan, J. L., et al. (2016). APC/C and SCF(cyclin F) constitute a reciprocal feedback circuit controlling S-phase entry. Cell Reports, 16(12), 3359–3372. https://doi.org/10.1016/j.celrep.2016.08.058.
Cizmecioglu, O., Arnold, M., Bahtz, R., Settele, F., Ehret, L., Haselmann-Weiß, U., et al. (2010). Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. Journal of Cell Biology. https://doi.org/10.1083/jcb.201007107.
Clausen, T. H., Lamark, T., Isakson, P., Finley, K., Larsen, K. B., Brech, A., et al. (2010). p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy, 6, 330–344. https://doi.org/10.4161/auto.6.3.11226.
Cohen-Katsenelson, K., Wasserman, T., Khateb, S., Whitmarsh, A. J., & Aronheim, A. (2011). Docking interactions of the JNK scaffold protein WDR62. The Biochemical Journal, 439(3), 381–390. https://doi.org/10.1042/BJ20110284.
Colombo, J., Kannass, K. N., Shaddy, D. J., Kundurthi, S., Maikranz, J. M., Anderson, C. J., et al. (2004). Maternal DHA and the development of attention in infancy and toddlerhood. Child Development, 75, 1254–1267.
Corson, T. W., Chang, Q. Z., Lau, S. K., Shepherd, F. A., Tsao, M. S., & Gallie, B. L. (2007). KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clinical Cancer Research. https://doi.org/10.1158/1078-0432.CCR-07-0393.
Corson, T. W., Huang, A., Tsao, M. S., & Gallie, B. L. (2005). KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene. https://doi.org/10.1038/sj.onc.1208641.
Cowie, V. (1960). The genetics and sub-classification of microcephaly. Journal of Intellectual Disability Research. https://doi.org/10.1111/j.1365-2788.1960.tb00751.x.
Cristofoli, F., De Keersmaecker, B., De Catte, L., Vermeesch, J. R., & Van Esch, H. (2017). Novel STIL compound heterozygous mutations cause severe fetal microcephaly and centriolar lengthening. Molecular Syndromology. https://doi.org/10.1159/000479666.
Cui, F., Hu, J., Xu, Z., Tan, J., & Tang, H. (2019). Overexpression of ncaph is upregulated and predicts a poor prognosis in prostate cancer. Oncology Letters, 17, 5768–5776. https://doi.org/10.3892/ol.2019.10260.
D'Avino, P. P. (2017). Citron kinase—Renaissance of a neglected mitotic kinase. Journal of Cell Science, 130, 1701–1708. https://doi.org/10.1242/jcs.200253.
D'Avino, P. P., & Capalbo, L. (2016). Regulation of midbody formation and function by mitotic kinases. Seminars in Cell and Developmental Biology, 53, 57–63.
Debec, A., Sullivan, W., & Bettencourt-Dias, M. (2010). Centrioles: Active players or passengers during mitosis? Cellular and Molecular Life Sciences, 67, 2173–2194.
Degrassi, F., Damizia, M., & Lavia, P. (2019). The mitotic apparatus and kinetochores in microcephaly and neurodevelopmental diseases. Cells, 9, 49. https://doi.org/10.3390/cells9010049.
Di Cunto, F., Calautti, E., Hsiao, J., Ong, L., Topley, G., Turco, E., et al. (1998). Citron Rho-interacting kinase, a novel tissue-specific Ser/Thr kinase encompassing the Rho-Rac-binding protein citron. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.273.45.29706.
Di Cunto, F., Hirsch, E., Broccoli, V., Bulfone, A., Migheli, A., Atzori, C., et al. (2000). Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron, 28(1), 115–127.
Dinçer, T., Yorgancıoğlu-Budak, G., Ölmez, A., Er, İ., Dodurga, Y., Özdemir, Ö. M. A., et al. (2017). Analysis of centrosome and DNA damage response in PLK4 associated Seckel syndrome. European Journal of Human Genetics. https://doi.org/10.1038/ejhg.2017.120.
Ding, W., Wu, Q., Sun, L., Pan, N. C., & Wang, X. (2019). Cenpj regulates cilia disassembly and neurogenesis in the developing mouse cortex. Journal of Neuroscience. https://doi.org/10.1523/JNEUROSCI.1849-18.2018.
DiStasio, A., Driver, A., Sund, K., Donlin, M., Muraleedharan, R. M., Pooya, S., et al. (2017). Copb2 is essential for embryogenesis and hypomorphic mutations cause human microcephaly. Human Molecular Genetics, 26, 4836–4848. https://doi.org/10.1093/hmg/ddx362.
Dungrawala, H., Rose, K. L., Bhat, K. P., Mohni, K. N., Glick, G. G., Couch, F. B., et al. (2015). The replication checkpoint prevents two types of fork collapse without regulating replisome stability. Molecular Cell. https://doi.org/10.1016/j.molcel.2015.07.030.
Eda, M., Yonemura, S., Kato, T., Watanabe, N., Ishizaki, T., Madaule, P., et al. (2001). Rho-dependent transfer of citron-kinase to the cleavage furrow of dividing cells. Journal of Cell Science, 114(18), 3273–3284.
Erez, A., Castiel, A., Trakhtenbrot, L., Perelman, M., Rosenthal, E., Goldstein, I., et al. (2007). The SIL gene is essential for mitotic entry and survival of cancer cells. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-07-0064.
Erez, A., Perelman, M., Hewitt, S. M., Cojacaru, G., Goldberg, I., Shahar, I., et al. (2004). Sil overexpression in lung cancer characterizes tumors with increased mitotic activity. Oncogene, 23, 5371–5377. https://doi.org/10.1038/sj.onc.1207685.
Espeut, J., Gaussen, A., Bieling, P., Morin, V., Prieto, S., Fesquet, D., et al. (2008). Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Molecular Cell. https://doi.org/10.1016/j.molcel.2008.01.004.
Faheem, M., Naseer, M. I., Rasool, M., Chaudhary, A. G., Kumosani, T. A., Ilyas, A. M., et al. (2015). Molecular genetics of human primary microcephaly: An overview. BMC Medical Genomics, 8, S4.
Farag, H. G., Froehler, S., Oexle, K., Ravindran, E., Schindler, D., Staab, T., et al. (2013). Abnormal centrosome and spindle morphology in a patient with autosomal recessive primary microcephaly type 2 due to compound heterozygous WDR62 gene mutation. Orphanet J Rare Dis, 8, 178. https://doi.org/10.1186/1750-1172-8-178.
Ferguson, K. L., Callaghan, S. M., O'Hare, M. J., Park, D. S., & Slack, R. S. (2000). The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. Journal of Biological Chemistry, 275, 33593–33600. https://doi.org/10.1074/jbc.M004879200.
Filimonenko, M., Isakson, P., Finley, K. D., Anderson, M., Jeong, H., Melia, T. J., et al. (2010). The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Molecular Cell. https://doi.org/10.1016/j.molcel.2010.04.007.
Fish, J. L., Kosodo, Y., Enard, W., Pääbo, S., & Huttner, W. B. (2006). Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.0604066103.
Fisher, C. L., & Fisher, A. G. (2011). Chromatin states in pluripotent, differentiated, and reprogrammed cells. Current Opinion in Genetics and Development, 21(2), 140–146.
Fong, K. W., Hau, S. Y., Kho, Y. S., Jia, Y., He, L., & Qi, R. Z. (2009). Interaction of CDK5RAP2 with EB1 to track growing microtubule tips and to regulate microtubule dynamics. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E09-01-0009.
Frantzi, M., Zoidakis, J., Papadopoulos, T., Zürbig, P., Katafigiotis, I., Stravodimos, K., et al. (2013). IMAC fractionation in combination with LC-MS reveals H2B and NIF-1 peptides as potential bladder cancer biomarkers. Journal of Proteome Research. https://doi.org/10.1021/pr400255h.
Fujikura, K., Setsu, T., Tanigaki, K., Abe, T., Kiyonari, H., Terashima, T., et al. (2013). Kif14 mutation causes severe brain malformation and hypomyelination. PLoS ONE. https://doi.org/10.1371/journal.pone.0053490.
Fujimori, A., Itoh, K., Goto, S., Hirakawa, H., Wang, B., Kokubo, T., et al. (2014). Disruption of Aspm causes microcephaly with abnormal neuronal differentiation. Brain and Development. https://doi.org/10.1016/j.braindev.2013.10.006.
Fujimori, A., Yaoi, T., Ogi, H., Wang, B., Suetomi, K., Sekine, E., et al. (2008). Ionizing radiation downregulates ASPM, a gene responsible for microcephaly in humans. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2008.02.149.
Fukagawa, T., & Earnshaw, W. C. (2014). The centromere: Chromatin foundation for the kinetochore machinery. Developmental Cell, 30(5), 496–508.
Fukasawa, K. (2005). Centrosome amplification, chromosome instability and cancer development. Cancer Letters, 230(1), 6–19.
Gai, M., Bianchi, F. T., Vagnoni, C., Vernì, F., Bonaccorsi, S., Pasquero, S., et al. (2016). ASPM and CITK regulate spindle orientation by affecting the dynamics of astral microtubules. EMBO reports, 18, 1870–1870. https://doi.org/10.15252/embr.201745023.
Gao, C., & Chen, Y. G. (2010). Dishevelled: The hub of Wnt signaling. Cellular Signalling, 22(5), 717–727.
Garapaty, S., Xu, C. F., Trojer, P., Mahajan, M. A., Neubert, T. A., & Samuels, H. H. (2009). Identification and characterization of a novel nuclear protein complex involved in nuclear hormone receptor-mediated gene regulation. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M805872200.
Garcez, P. P., Diaz-Alonso, J., Crespo-Enriquez, I., Castro, D., Bell, D., & Guillemot, F. (2015). Cenpj/CPAP regulates progenitor divisions and neuronal migration in the cerebral cortex downstream of Ascl1. Nature Communications. https://doi.org/10.1038/ncomms7474.
Garshasbi, M., Motazacker, M. M., Kahrizi, K., Behjati, F., Abedini, S. S., Nieh, S. E., et al. (2006). SNP array-based homozygosity mapping reveals MCPH1 deletion in family with autosomal recessive mental retardation and mild microcephaly. Human Genetics. https://doi.org/10.1007/s00439-005-0104-y.
Gay, S., & Foiani, M. (2015). Nuclear envelope and chromatin, lock and key of genome integrity. International Review of Cell and Molecular Biology. https://doi.org/10.1016/bs.ircmb.2015.03.001.
Genin, A., Desir, J., Lambert, N., Biervliet, M., Van der Aa, N., Pierquin, G., et al. (2012). Kinetochore KMN network gene CASC5 mutated in primary microcephaly. Human Molecular Genetics. https://doi.org/10.1093/hmg/dds386.
Gönczy, P. (2012). Towards a molecular architecture of centriole assembly. Nature Reviews Molecular Cell Biology, 13(7), 425–435.
Gopalakrishnan, J., Guichard, P., Smith, A. H., Schwarz, H., Agard, D. A., Marco, S., et al. (2010). Self-assembling SAS-6 multimer is a core centriole building block. Journal of Biological Chemistry, 285, 8759–8770. https://doi.org/10.1074/jbc.M109.092627.
Gruber, R. (2011). Function of MCPH1 in neurogenesis dissertation (pp. 1–164).
Gruber, R., Zhou, Z., Sukchev, M., Joerss, T., Frappart, P. O., & Wang, Z. Q. (2011). MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nature Cell Biology. https://doi.org/10.1038/ncb2342.
Gruneberg, U., Neef, R., Li, X., Chan, E. H. Y. Y., Chalamalasetty, R. B., Nigg, E. A., et al. (2006). KIF14 and citron kinase act together to promote efficient cytokinesis. Journal of Cell Biology, 172, 363–372. https://doi.org/10.1083/jcb.200511061.
Guemez-Gamboa, A., Nguyen, L. N., Yang, H., Zaki, M. S., Kara, M., Ben-Omran, T., et al. (2015). Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nature Genetics. https://doi.org/10.1038/ng.3311.
Guernsey, D. L., Jiang, H., Hussin, J., Arnold, M., Bouyakdan, K., Perry, S., et al. (2010). Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. American Journal of Human Genetics. https://doi.org/10.1016/j.ajhg.2010.06.003.
Guesnet, P., & Alessandri, J. M. (2011). Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—Implications for dietary recommendations. Biochimie, 93(1), 7–12.
Guo, Y., Kim, C., Ahmad, S., Zhang, J., & Mao, Y. (2012). CENP-E-dependent bubr1 autophosphorylation enhances chromosome alignment and the mitotic checkpoint. Journal of Cell Biology, 198, 205–217. https://doi.org/10.1083/jcb.201202152.
Gustafsson, B. M., Mattsson, K., Bogdanovic, G., Leijonhufvud, G., Honkaniemi, E., Ramme, K., et al. (2018). Origins of STIL-TAL1 fusion genes in children who later developed paediatric T-cell acute lymphoblastic leukaemia: An investigation of neonatal blood spots. Pediatric Blood and Cancer. https://doi.org/10.1002/pbc.27310.
Habedanck, R., Stierhof, Y. D., Wilkinson, C. J., & Nigg, E. A. (2005). The Polo kinase Plk4 functions in centriole duplication. Nature Cell Biology. https://doi.org/10.1038/ncb1320.
Hara, M., & Fukagawa, T. (2018). Kinetochore assembly and disassembly during mitotic entry and exit. Current Opinion in Cell Biology, 52, 73–81. https://doi.org/10.1016/j.ceb.2018.02.005.
Harel, T., Quek, D. Q. Y., Wong, B. H., Cazenave-Gassiot, A., Wenk, M. R., Fan, H., et al. (2018). Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics, 19, 227–235. https://doi.org/10.1007/s10048-018-0556-6.
Hasenpusch-Theil, K., West, S., Kelman, A., Kozic, Z., Horrocks, S., McMahon, A. P., et al. (2018). Gli3 controls the onset of cortical neurogenesis by regulating the radial glial cell cycle through Cdk6 expression. Development, 145(17), 163147. https://doi.org/10.1242/dev.163147.
Hatch, E. M., Kulukian, A., Holland, A. J., Cleveland, D. W., & Stearns, T. (2010). Cep152 interacts with Plk4 and is required for centriole duplication. Journal of Cell Biology. https://doi.org/10.1083/jcb.201006049.
Heird, W. C., & Lapillonne, A. (2005). The role of essential fatty acids in development. Annual Review of Nutrition. https://doi.org/10.1146/annurev.nutr.24.012003.132254.
Hetzer, M. W., Walther, T. C., & Mattaj, I. W. (2005). Pushing the envelope: Structure, function, and dynamics of the nuclear periphery. Annual Review of Cell and Developmental Biology. https://doi.org/10.1146/annurev.cellbio.21.090704.151152.
Hirano, T. (2012). Condensins: Universal organizers of chromosomes with diverse functions. Genes and Development, 26(15), 1659–1678.
Hirano, T. (2016). Condensin-based chromosome organization from bacteria to vertebrates. Cell, 164(5), 847–857.
Hirano, T., Kobayashi, R., & Hirano, M. (1997). Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell, 5, 10. https://doi.org/10.1016/S0092-8674(00)80233-0.
Hirota, T., Gerlich, D., Koch, B., Ellenberg, J., & Peters, J. M. (2004). Distinct functions of condensin I and II in mitotic chromosome assembly. Journal of Cell Science. https://doi.org/10.1242/jcs.01604.
Horvath, S., Zhang, B., Carlson, M., Lu, K. V., Zhu, S., Felciano, R. M., et al. (2006). Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.0608396103.
Hsu, C. C., Liao, W. Y., Chan, T. S., Chen, W. Y., Lee, C. T., Shan, Y. S., et al. (2019). The differential distributions of ASPM isoforms and their roles in Wnt signaling, cell cycle progression, and pancreatic cancer prognosis. Journal of Pathology, 249, 498–508. https://doi.org/10.1002/path.5341.
Huang, L., Wang, T., Wang, F., Hu, X., Zhan, G., Jin, X., et al. (2020). NUP37 silencing induces inhibition of cell proliferation, G1 phase cell cycle arrest and apoptosis in non-small cell lung cancer cells. Pathology Research and Practice, 216, 152836. https://doi.org/10.1016/j.prp.2020.152836.
Hung, L. Y., Chen, H. L., Chang, C. W., Li, B. R., & Tang, T. K. (2004). Identification of a novel microtubule-destabilizing motif in CPAP that binds to tubulin heterodimers and inhibits microtubule assembly. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E04-02-0121.
Hung, L.-Y., Tang, C.-J. C., & Tang, T. K. (2000). Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.20.20.7813-7825.2000..
Hung, P. F., Hong, T. M., Hsu, Y. C., Chen, H. Y., Chang, Y. L., Wu, C. T., et al. (2013). The motor protein KIF14 inhibits tumor growth and cancer metastasis in lung adenocarcinoma. PLoS ONE. https://doi.org/10.1371/journal.pone.0061664.
Hussain, M. S., Baig, S. M., Neumann, S., Nürnberg, G., Farooq, M., Ahmad, I., et al. (2012). A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. American Journal of Human Genetics. https://doi.org/10.1016/j.ajhg.2012.03.016.
Hussain, M. S., Baig, S. M., Neumann, S., Peche, V. S., Szczepanski, S., Nürnberg, G., et al. (2013). CDK6 associates with the centrosome during mitosis and is mutated in a large pakistani family with primary microcephaly. Human Molecular Genetics, 22, 5199–5214. https://doi.org/10.1093/hmg/ddt374.
Ibarra, A., & Hetzer, M. W. (2015). Nuclear pore proteins and the control of genome functions. Genes and Development, 29(4), 337–349.
Insolera, R., Bazzi, H., Shao, W., Anderson, K. V., & Shi, S. H. (2014). Cortical neurogenesis in the absence of centrioles. Nature Neuroscience. https://doi.org/10.1038/nn.3831.
Issa, L., Kraemer, N., Rickert, C. H., Sifringer, M., Ninnemann, O., Stoltenburg-Didinger, G., et al. (2013a). CDK5RAP2 expression during murine and human brain development correlates with pathology in primary autosomal recessive microcephaly. Cerebral Cortex. https://doi.org/10.1093/cercor/bhs212.
Issa, L., Mueller, K., Seufert, K., Kraemer, N., Rosenkotter, H., Ninnemann, O., et al. (2013b). Clinical and cellular features in patients with primary autosomal recessive microcephaly and a novel CDK5RAP2 mutation. Orphanet Journal of Rare Diseases, 8, 1–14. https://doi.org/10.1186/1750-1172-8-59.
Jana, S. C., Marteil, G., & Bettencourt-Dias, M. (2014). Mapping molecules to structure: Unveiling secrets of centriole and cilia assembly with near-atomic resolution. Current Opinion in Cell Biology, 26, 96–106.
Jayaraman, D., Kodani, A., Gonzalez, D. M., Mancias, J. D., Mochida, G. H., Vagnoni, C., et al. (2016). Microcephaly proteins Wdr62 and Aspm define a mother centriole complex regulating centriole biogenesis, apical complex, and cell fate. Neuron, 92, 813–828. https://doi.org/10.1016/j.neuron.2016.09.056.
Jeffers, L. J., Coull, B. J., Stack, S. J., & Morrison, C. G. (2008). Distinct BRCT domains in Mcph1/Brit1 mediate ionizing radiation-induced focus formation and centrosomal localization. Oncogene. https://doi.org/10.1038/sj.onc.1210595.
Jena, N., Sheng, J., Hu, J. K., Li, W., Zhou, W., Lee, G., et al. (2016). CDK6-mediated repression of CD25 is required for induction and maintenance of Notch1-induced T-cell acute lymphoblastic leukemia. Leukemia, 30(5), 1033–1043. https://doi.org/10.1038/leu.2015.353.
Johnson, C. A., Wright, C. E., & Ghashghaei, H. T. (2017). Regulation of cytokinesis during corticogenesis: focus on the midbody. FEBS Letters, 591, 4009–4026. https://doi.org/10.1002/1873-3468.12676.
Kadir, R., Harel, T., Markus, B., Perez, Y., Bakhrat, A., Cohen, I., et al. (2016). ALFY-controlled DVL3 autophagy regulates Wnt signaling, determining human brain size. PLoS Genetics, 12, 1–21. https://doi.org/10.1371/journal.pgen.1005919.
Kalay, E., Yigit, G., Aslan, Y., Brown, K. E., Pohl, E., Bicknell, L. S., et al. (2011). CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nature Genetics. https://doi.org/10.1038/ng.725.
Kaufmann, T., Kukolj, E., Brachner, A., Beltzung, E., Bruno, M., Kostrhon, S., et al. (2016). SIRT2 regulates nuclear envelope reassembly through ANKLE2 deacetylation. Journal of Cell Science. https://doi.org/10.1242/jcs.192633.
Keller, D., Orpinell, M., Olivier, N., Wachsmuth, M., Mahen, R., Wyss, R., et al. (2014). Mechanisms of HsSAS-6 assembly promoting centriole formation in human cells. Journal of Cell Biology. https://doi.org/10.1083/jcb.201307049.
Khan, M. A., Rupp, V. M., Orpinell, M., Hussain, M. S., Altmüller, J., Steinmetz, M. O., et al. (2014). A missense mutation in the PISA domain of HsSAS-6 causes autosomal recessive primary microcephaly in a large consanguineous pakistani family. Human Molecular Genetics, 23, 5940–5949. https://doi.org/10.1093/hmg/ddu318.
Kim, H. T., Lee, M. S., Choi, J. H., Jung, J. Y., Ahn, D. G., Yeo, S. Y., et al. (2011). The microcephaly gene aspm is involved in brain development in zebrafish. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2011.05.056.
Kishi, Y., & Gotoh, Y. (2018). Regulation of chromatin structure during neural development. Frontiers in Neuroscience, 12, 874.
Kitagawa, D., Kohlmaier, G., Keller, D., Strnad, P., Balestra, F. R., Flückiger, I., et al. (2011). Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. Journal of Cell Science. https://doi.org/10.1242/jcs.089888.
Kiyomitsu, T., Murakami, H., & Yanagida, M. (2011). Protein interaction domain mapping of human kinetochore protein blinkin reveals a consensus motif for binding of spindle assembly checkpoint proteins Bub1 and BubR1. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.00815-10.
Kleylein-Sohn, J., Westendorf, J., Le Clech, M., Habedanck, R., Stierhof, Y. D., & Nigg, E. A. (2007). Plk4-induced centriole biogenesis in human cells. Developmental Cell. https://doi.org/10.1016/j.devcel.2007.07.002.
Kodani, A., Yu, T. W., Johnson, J. R., Jayaraman, D., Johnson, T. L., Al-Gazali, L., et al. (2015). Centriolar satellites assemble centrosomal microcephaly proteins to recruit CDK2 and promote centriole duplication. eLife. https://doi.org/10.7554/eLife.07519.
Kumar, A., Girimaji, S. C., Duvvari, M. R., & Blanton, S. H. (2008). Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. American Journal of Human Genetics. https://doi.org/10.1016/j.ajhg.2009.01.017.
Kuwahara, A., Hirabayashi, Y., Knoepfler, P. S., Taketo, M. M., Sakai, J., Kodama, T., et al. (2010). Wnt signaling and its downstream target N-myc regulate basal progenitors in the developing neocortex. Development. https://doi.org/10.1242/dev.046417.
Lecland, N., Debec, A., Delmas, A., Moutinho-Pereira, S., Malmanche, N., Bouissou, A., et al. (2013). Establishment and mitotic characterization of new Drosophila acentriolar cell lines from DSas-4 mutant. Biology Open. https://doi.org/10.1242/bio.20133327.
Lee, I. W., Jo, Y. J., Jung, S. M., Wang, H. Y., Kim, N. H., & Namgoong, S. (2018). Distinct roles of Cep192 and Cep152 in acentriolar MTOCs and spindle formation during mouse oocyte maturation. FASEB Journal, 32, 625–638. https://doi.org/10.1096/fj.201700559RR.
Leidel, S., Delattre, M., Cerutti, L., Baumer, K., & Gönczy, P. (2005). SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nature Cell Biology. https://doi.org/10.1038/ncb1220.
Letourneur, F., Gaynor, E. C., Hennecke, S., Démollière, C., Duden, R., Emr, S. D., et al. (1994). Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. https://doi.org/10.1016/0092-8674(94)90011-6.
Li, H., Bielas, S. L., Zaki, M. S., Ismail, S., Farfara, D., Um, K., et al. (2016). Biallelic mutations in citron kinase link mitotic cytokinesis to human primary microcephaly. American Journal of Human Genetics, 99, 501–510. https://doi.org/10.1016/j.ajhg.2016.07.004.
Liang, Y. L., Gao, H., Lin, S. Y., Peng, G., Huang, X. X., Zhang, P. M., et al. (2010). BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genetics, 6(1), e1000826. https://doi.org/10.1371/journal.pgen.1000826.
Lim, N. R., Shohayeb, B., Zaytseva, O., Mitchell, N., Millard, S. S., Ng, D. C. H., et al. (2017). Glial-specific functions of microcephaly protein WDR62 and interaction with the mitotic kinase AURKA are essential for Drosophila brain growth. Stem Cell Reports, 9(1), 32–41. https://doi.org/10.1016/j.stemcr.2017.05.015.
Lim, N. R., Yeap, Y. Y., Ang, C. S., Williamson, N. A., Bogoyevitch, M. A., Quinn, L. M., et al. (2016). Aurora A phosphorylation of WD40-repeat protein 62 in mitotic spindle regulation. Cell Cycle, 15(3), 413–424. https://doi.org/10.1080/15384101.2015.1127472.
Lim, N. R., Yeap, Y. Y., Zhao, T. T., Yip, Y. Y., Wong, S. C., Xu, D., et al. (2015). Opposing roles for JNK and aurora A in regulating the association of WDR62 with spindle microtubules. Journal of Cell Science, 128(3), 527–540. https://doi.org/10.1242/jcs.157537.
Lin, S. Y., Pan, H. W., Liu, S. H., Jeng, Y. M., Hu, F. C., Peng, S. Y., et al. (2008). ASPM is a novel marker for vascular invasion, early recurrence, and poor prognosis of hepatocellular carcinoma. Clinical Cancer Research. https://doi.org/10.1158/1078-0432.CCR-07-5262.
Lin, Y. C., Chang, C. W., Hsu, W. B., Tang, C. J. C., Lin, Y. N., Chou, E. J., et al. (2013). Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. EMBO Journal. https://doi.org/10.1038/emboj.2013.56.
Liu, D., Ding, X., Du, J., Cai, X., Huang, Y., Ward, T., et al. (2007). Human NUF2 interacts with centromere-associated protein E and is essential for a stable spindle microtubule-kinetochore attachment. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M609026200.
Liu, X., Zhou, Z. W., & Wang, Z. Q. (2016). The DNA damage response molecule MCPH1 in brain development and beyond. Acta Biochimica et Biophysica Sinica, 48, 678–685. https://doi.org/10.1093/abbs/gmw048.
Liu, X., Zong, W., Li, T., Wang, Y., Xu, X., Zhou, Z. W., et al. (2017). The E3 ubiquitin ligase APC/C(C)(dh1) degrades MCPH1 after MCPH1-betaTrCP2-Cdc25A-mediated mitotic entry to ensure neurogenesis. EMBO Journal, 36(24), 3666–3681. https://doi.org/10.15252/embj.201694443.
Lizarraga, S. B., Margossian, S. P., Harris, M. H., Campagna, D. R., Han, A. P., Blevins, S., et al. (2010). Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. https://doi.org/10.1242/dev.040410.
Loïodice, I., Alves, A., Rabut, G., Van Overbeek, M., Ellenberg, J., Sibarita, J. B., et al. (2004). The entire Nup107-160 complex, including three new members, is targeted as one entity to kinetochores in mitosis. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E03-12-0878.
Lucas, J. J., Domenico, J., & Gelfand, E. W. (2004). Cyclin-dependent kinase 6 inhibits proliferation of human mammary epithelial cells. Molecular Cancer Research, 2, 105–114.
Luo, L., & Kessel, M. (2004). Geminin coordinates cell cycle and developmental control. Cell Cycle, 3, 709–712.
Luo, X., Liu, Y., Feng, W., Lei, L., Du, Y., Wu, J., et al. (2017). NUP37, a positive regulator of YAP/TEAD signaling, promotes the progression of hepatocellular carcinoma. Oncotarget, 8, 98004–98013. https://doi.org/10.18632/oncotarget.20336.
Ma, D. K., Marchetto, M. C., Guo, J. U., Ming, G. L., Gage, F. H., & Song, H. (2010). Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nature Neuroscience, 13, 1388–1394.
Madaule, P., Eda, M., Watanabe, N., Fujisawa, K., Matsuoka, T., Bito, H., et al. (1998). Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. https://doi.org/10.1038/28873.
Mai, L., Yi, F., Gou, X., Zhang, J., Wang, C., Liu, G., et al. (2014). The overexpression of MCPH1 inhibits cell growth through regulating cell cycle-related proteins and activating cytochrome c-caspase 3 signaling in cervical cancer. Molecular and Cellular Biochemistry. https://doi.org/10.1007/s11010-014-2022-6.
Markowski, J., Tyszkiewicz, T., Jarza̧b, M., Oczko-Wojciechowska, M., Gierek, T., Witkowska, M., et al. (2009). Metal-proteinase ADAM12, kinesin 14 and checkpoint suppressor 1 as new molecular markers of laryngeal carcinoma. European Archives of Oto-Rhino-Laryngology, 266(10), 1501–1507.
Martin, C.-A. A., Murray, J. E., Carroll, P., Leitch, A., Mackenzie, K. J., Halachev, M., et al. (2016). Mutations in genes encoding condensin complex proteins cause microcephaly through decatenation failure at mitosis. Genes and Development. https://doi.org/10.1101/gad.286351.116.
Martinez, M. (1996). Docosahexaenoic acid therapy in docosahexaenoic acid-deficient patients with disorders of peroxisomal biogenesis. Lipids. https://doi.org/10.1007/bf02637067.
Marx, A., Hoenger, A., & Mandelkow, E. (2009). Structures of kinesin motor proteins. Cell Motility and the Cytoskeleton, 66(11), 958–966.
Matushansky, I., Radparvar, F., & Skoultchi, A. I. (2003). CDK6 blocks differentiation: Coupling cell proliferation to the block to differentiation in leukemic cells. Oncogene. https://doi.org/10.1038/sj.onc.1206484.
McKenzie, C., Bassi, Z. I., Debski, J., Gottardo, M., Callaini, G., Dadlez, M., et al. (2016). Cross-regulation between aurora B and citron kinase controls midbody architecture in cytokinesis. Open Biology. https://doi.org/10.1098/rsob.160019.
McNamara, R. K. (2010). DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. The Journal of Nutrition. https://doi.org/10.3945/jn.109.113233.
Meyerson, M., & Harlow, E. (1994). Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.14.3.2077.
Mi, Y., Sun, C., Wei, B., Sun, F., Guo, Y., Hu, Q., et al. (2018). Coatomer subunit beta 2 (COPB2), identified by label-free quantitative proteomics, regulates cell proliferation and apoptosis in human prostate carcinoma cells. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2017.11.040.
Mi, Y., Yu, M., Zhang, L., Sun, C., Wei, B., Ding, W., et al. (2016). COPB2 is upregulated in prostate cancer and regulates PC-3 cell proliferation, cell cycle, and apoptosis. Archives of Medical Research, 47, 411–418. https://doi.org/10.1016/j.arcmed.2016.09.005.
Mirzaa, G. M., Vitre, B., Carpenter, G., Abramowicz, I., Gleeson, J. G., Paciorkowski, A. R., et al. (2014). Mutations in CENPE define a novel kinetochore-centromeric mechanism for microcephalic primordial dwarfism. Human Genetics. https://doi.org/10.1007/s00439-014-1443-3.
Miyamoto, T., Akutsu, S. N., Fukumitsu, A., Morino, H., Masatsuna, Y., Hosoba, K., et al. (2017). PLK1-mediated phosphorylation of WDR62/MCPH2 ensures proper mitotic spindle orientation. Human Molecular Genetics, 26, 4429–4440. https://doi.org/10.1093/hmg/ddx330.
Moawia, A., Shaheen, R., Rasool, S., Waseem, S. S., Ewida, N., Budde, B., et al. (2017). Mutations of KIF14 cause primary microcephaly by impairing cytokinesis. Annals of Neurology, 82, 562–577. https://doi.org/10.1002/ana.25044.
Molnar, Z., Metin, C., Stoykova, A., Tarabykin, V., Price, D. J., Francis, F., et al. (2006). Comparative aspects of cerebral cortical development. European Journal of Neuroscience, 23(4), 921–934. https://doi.org/10.1111/j.1460-9568.2006.04611.x.
Mouden, C., De Tayrac, M., Dubourg, C., Rose, S., Carré, W., Hamdi-Rozé, H., et al. (2015). Homozygous STIL mutation causes holoprosencephaly and microcephaly in two siblings. PLoS ONE. https://doi.org/10.1371/journal.pone.0117418.
Musacchio, A. (2015). The molecular biology of spindle assembly checkpoint signaling dynamics. Current Biology, 25(20), R1002–R1018.
Naveed, M., Kazmi, S. K., Amin, M., Asif, Z., Islam, U., Shahid, K., et al. (2018). Comprehensive review on the molecular genetics of autosomal recessive primary microcephaly (MCPH). Genetics Research, 100, e7. https://doi.org/10.1017/S0016672318000046.
Neitzel, H., Neumann, L. M., Schindler, D., Wirges, A., Tönnies, H., Trimborn, M., et al. (2002). Premature chromosome condensation in humans associated with microcephaly and mental retardation: A novel autosomal recessive condition. American Journal of Human Genetics. https://doi.org/10.1086/339518.
Ng, R. K., & Gurdon, J. B. (2008). Epigenetic inheritance of cell differentiation status. Cell Cycle, 7(9), 1173–1177.
Nicholas, A. K., Khurshid, M., Désir, J., Carvalho, O. P., Cox, J. J., Thornton, G., et al. (2010). WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nature Genetics. https://doi.org/10.1038/ng.682.
Nicholas, A. K., Swanson, E. A., Cox, J. J., Karbani, G., Malik, S., Springell, K., et al. (2009). The molecular landscape of ASPM mutations in primary microcephaly. Journal of Medical Genetics. https://doi.org/10.1136/jmg.2008.062380.
Nigg, E. A., Čajánek, L., & Arquint, C. (2014). The centrosome duplication cycle in health and disease. FEBS Letters, 588(15), 2366–2372.
Nigg, E. A., & Raff, J. W. (2009). Centrioles, centrosomes, and cilia in health and disease. Cell, 139, 663–678.
Noatynska, A., Gotta, M., & Meraldi, P. (2012). Mitotic spindle (DIS)orientation and DISease: cause or consequence? Journal of Cell Biology, 199(7), 1025–1035. https://doi.org/10.1083/jcb.201209015.
Ogasawara, T., Kawaguchi, H., Jinno, S., Hoshi, K., Itaka, K., Takato, T., et al. (2004). Bone morphogenetic protein 2-induced osteoblast differentiation requires Smad-mediated down-regulation of Cdk6. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.24.15.6560-6568.2004.
Ohta, S., Wood, L., Toramoto, I., Yagyu, K. I., Fukagawa, T., & Earnshaw, W. C. (2015). CENP-32 is required to maintain centrosomal dominance in bipolar spindle assembly. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E14-09-1366.
Omer Javed, A., Li, Y., Muffat, J., Su, K. C., Cohen, M. A., Lungjangwa, T., et al. (2018). Microcephaly modeling of kinetochore mutation reveals a brain-specific phenotype. Cell Reports. https://doi.org/10.1016/j.celrep.2018.09.032.
Pai, V. C., Hsu, C. C., Chan, T. S., Liao, W. Y., Chuu, C. P., Chen, W. Y., et al. (2019). ASPM promotes prostate cancer stemness and progression by augmenting Wnt-Dvl-3-β-catenin signaling. Oncogene, 38, 1340–1353. https://doi.org/10.1038/s41388-018-0497-4.
Pallavicini, G., Sgro, F., Garello, F., Falcone, M., Bitonto, V., Berto, G. E., et al. (2018). Inactivation of citron kinase inhibits medulloblastoma progression by inducing apoptosis and cell senescence. Cancer Research, 78, 4599–4612. https://doi.org/10.1158/0008-5472.CAN-17-4060.
Papp, B., & Müller, J. (2006). Histone trimethylation and the maintenance of transcriptional ON and OFF states by trxG and PcG proteins. Genes and Development. https://doi.org/10.1101/gad.388706.
Passemard, S., Verloes, A., Billette de Villemeur, T., Boespflug-Tanguy, O., Hernandez, K., Laurent, M., et al. (2016). Abnormal spindle-like microcephaly-associated (ASPM) mutations strongly disrupt neocortical structure but spare the hippocampus and long-term memory. Cortex, 74, 158–176. https://doi.org/10.1016/j.cortex.2015.10.010.
Patwardhan, D., Mani, S., Passemard, S., Gressens, P., & El Ghouzzi, V. (2018). STIL balancing primary microcephaly and cancer. Cell Death and Disease, 9, 65. https://doi.org/10.1038/s41419-017-0101-9.
Peel, N., Stevens, N. R., Basto, R., & Raff, J. W. (2007). Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Current Biology. https://doi.org/10.1016/j.cub.2007.04.036.
Perez, Y., Bar-yaacov, R., Kadir, R., Wormser, O., Shelef, I., Birk, O. S., et al. (2019). Mutations in the microtubule-associated protein MAP11 (C7orf43) cause microcephaly in humans and zebrafish. Brain, 11, 574–585. https://doi.org/10.1093/brain/awz004.
Petrovic, A., Pasqualato, S., Dube, P., Krenn, V., Santaguida, S., Cittaro, D., et al. (2010). The MIS12 complex is a protein interaction hub for outer kinetochore assembly. Journal of Cell Biology. https://doi.org/10.1083/jcb.201002070.
Pu, X., Wang, J., Li, W., Fan, W., Wang, L., Mao, Y., et al. (2018). COPB2 promotes cell proliferation and tumorigenesis through up-regulating YAP1 expression in lung adenocarcinoma cells. Biomedicine and Pharmacotherapy, 103, 373–380. https://doi.org/10.1016/j.biopha.2018.04.006.
Putkey, F. R., Cramer, T., Morphew, M. K., Silk, A. D., Johnson, R. S., McIntosh, J. R., et al. (2002). Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Developmental Cell. https://doi.org/10.1016/S1534-5807(02)00255-1.
Rabinowicz, N., Mangala, L. S., Brown, K. R., Checa-Rodriguez, C., Castiel, A., Moskovich, O., et al. (2017). Targeting the centriolar replication factor STIL synergizes with DNA damaging agents for treatment of ovarian cancer. Oncotarget. https://doi.org/10.18632/oncotarget.16068.
Rai, R., Dai, H., Multani, A. S., Li, K., Chin, K., Gray, J., et al. (2006). BRIT1 regulates early DNA damage response, chromosomal integrity, and cancer. Cancer Cell. https://doi.org/10.1016/j.ccr.2006.07.002.
Rai, R., Phadnis, A., Haralkar, S., Badwe, R. A. R. A., Dai, H., Li, K., et al. (2008). Differential regulation of centrosome integrity by DNA damage response proteins. Cell Cycle, 7, 2225–2233. https://doi.org/10.4161/cc.7.14.6303.
Raleigh, D. R., Choksi, P. K., Krup, A. L., Mayer, W., Santos, N., & Reiter, J. F. (2018). Hedgehog signaling drives medulloblastoma growth via CDK6. Journal of Clinical Investigation, 128(1), 120–124. https://doi.org/10.1172/JCI92710.
Rasika, S., Passemard, S., Verloes, A., Gressens, P., & Ghouzzi, V. E. (2019). Golgipathies in neurodevelopment: A new view of old defects. Developmental Neuroscience, 40(5–6), 396–416.
Renzova, T., Bohaciakova, D., Esner, M., Pospisilova, V., Barta, T., Hampl, A., et al. (2018). Inactivation of PLK4-STIL module prevents self-renewal and triggers p53-dependent differentiation in human pluripotent stem cells. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2018.08.008.
Rieder, C. L., Cole, R. W., Khodjakov, A., & Sluder, G. (1995). The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. Journal of Cell Biology. https://doi.org/10.1083/jcb.130.4.941.
Rodrigues-Martins, A., Bettencourt-Dias, M., Riparbelli, M., Ferreira, C., Ferreira, I., Callaini, G., et al. (2007a). DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Current Biology, 17, 1465–1472. https://doi.org/10.1016/j.cub.2007.07.034.
Rodrigues-Martins, A., Riparbelli, M., Callaini, G., Glover, D. M., & Bettencourt-Dias, M. (2007b). Revisiting the role of the mother centriole in centriole biogenesis. Science. https://doi.org/10.1126/science.1142950.
Rosti, R. O., Sotak, B. N., Bielas, S. L., Bhat, G., Silhavy, J. L., Aslanger, A. D., et al. (2017). Homozygous mutation in NUP107 leads to microcephaly with steroid-resistant nephrotic condition similar to Galloway-Mowat syndrome. Journal of Medical Genetics. https://doi.org/10.1136/jmedgenet-2016-104237.
Sankaran, D. G., Stemm-Wolf, A. J., & Pearson, C. G. (2019). CEP135 isoform dysregulation promotes centrosome amplification in breast cancer cells. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E18-10-0674.
Schmidt, T. I., Kleylein-Sohn, J., Westendorf, J., Le Clech, M., Lavoie, S. B., Stierhof, Y. D., et al. (2009). Control of centriole length by CPAP and CP110. Current Biology. https://doi.org/10.1016/j.cub.2009.05.016.
Sgrò, F., Bianchi, F. T., Falcone, M., Pallavicini, G., Gai, M., Chiotto, A. M. A., et al. (2016). Tissue-specific control of midbody microtubule stability by Citron kinase through modulation of TUBB3 phosphorylation. Cell Death and Differentiation. https://doi.org/10.1038/cdd.2015.142.
Shan, L., Zhao, M., Lu, Y., Ning, H., Yang, S., Song, Y., et al. (2019). CENPE promotes lung adenocarcinoma proliferation and is directly regulated by FOXM1. International Journal of Oncology. https://doi.org/10.3892/ijo.2019.4805.
Shi, L., Hu, E., Wang, Z., Liu, J., Li, J., Li, M., et al. (2017). Regional selection of the brain size regulating gene CASC5 provides new insight into human brain evolution. Human Genetics. https://doi.org/10.1007/s00439-016-1748-5.
Shi, L., Qalieh, A., Lam, M. M., Keil, J. M., & Kwan, K. Y. (2019). Robust elimination of genome-damaged cells safeguards against brain somatic aneuploidy following Knl1 deletion. Nature Communications. https://doi.org/10.1038/s41467-019-10411-w.
Shinmura, K., Kato, H., Kawanishi, Y., Igarashi, H., Inoue, Y., Yoshimura, K., et al. (2017). WDR62 overexpression is associated with a poor prognosis in patients with lung adenocarcinoma. Molecular Carcinogenesis, 56, 1984–1991. https://doi.org/10.1002/mc.22647.
Shinmura, K., Kato, H., Kawanishi, Y., Nagura, K., Kamo, T., Okubo, Y., et al. (2015). SASS6 overexpression is associated with mitotic chromosomal abnormalities and a poor prognosis in patients with colorectal cancer. Oncology Reports. https://doi.org/10.3892/or.2015.4014.
Shintomi, K., & Hirano, T. (2011). The relative ratio of condensing I to II determines chromosome shapes. Genes and Development. https://doi.org/10.1101/gad.2060311.
Simonsen, A., Birkeland, H. C. G., Gillooly, D. J., Mizushima, N., Kuma, A., Yoshimori, T., et al. (2004). Alfy, a novel FYVE-domain-containing protein associated with protein granules and autophagic membranes. Journal of Cell Science. https://doi.org/10.1242/jcs.01287.
Singh, P., Ramdas Nair, A., & Cabernard, C. (2014). The centriolar protein Bld10/Cep135 is required to establish centrosome asymmetry in Drosophila neuroblasts. Current Biology. https://doi.org/10.1016/j.cub.2014.05.050.
Snyers, L., Erhart, R., Laffer, S., Pusch, O., Weipoltshammer, K., & Schöfer, C. (2018). LEM4/ANKLE-2 deficiency impairs post-mitotic re-localization of BAF, LAP2α and LaminA to the nucleus, causes nuclear envelope instability in telophase and leads to hyperploidy in HeLa cells. European Journal of Cell Biology, 97, 63–74. https://doi.org/10.1016/j.ejcb.2017.12.001.
Song, B., Du, J., Song, D. F., Ren, J. C., & Feng, Y. (2018). Dysregulation of NCAPG, KNL1, miR-148a-3p, miR-193b-3p, and miR-1179 may contribute to the progression of gastric cancer. Biological Research. https://doi.org/10.1186/s40659-018-0192-5.
Sonnen, K. F., Gabryjonczyk, A. M., Anselm, E., Nigg, E. A., & Stierhof, Y. D. (2013). Human cep192 and cep152 cooperate in plk4 recruitment and centriole duplication. Journal of Cell Science. https://doi.org/10.1242/jcs.129502.
Sparmann, A., & Van Lohuizen, M. (2006). Polycomb silencers control cell fate, development and cancer. Nature Reviews Cancer, 6, 846–856. https://doi.org/10.1038/nrc1991.
Spinola, M., Falvella, F. S., Colombo, F., Sullivan, J. P., Shames, D. S., Girard, L., et al. (2010). MFSD2A is a novel lung tumor suppressor gene modulating cell cycle and matrix attachment. Molecular Cancer. https://doi.org/10.1186/1476-4598-9-62.
Strnad, P., Leidel, S., Vinogradova, T., Euteneuer, U., Khodjakov, A., & Gönczy, P. (2007). Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Developmental Cell, 13, 203–213. https://doi.org/10.1016/j.devcel.2007.07.004.
Stukenberg, P. T., & Burke, D. J. (2015). Connecting the microtubule attachment status of each kinetochore to cell cycle arrest through the spindle assembly checkpoint. Chromosoma, 124(4), 463–480.
Styers, M. L., O'Connor, A. K., Grabski, R., Cormet-Boyaka, E., & Sztul, E. (2008). Depletion of β-COP reveals a role for COP-I in compartmentalization of secretory compartments and in biosynthetic transport of caveolin-1. American Journal of Physiology-Cell Physiology. https://doi.org/10.1152/ajpcell.00010.2008.
Sukumaran, S. K., Stumpf, M., Salamon, S., Ahmad, I., Bhattacharya, K., Fischer, S., et al. (2017). CDK5RAP2 interaction with components of the Hippo signaling pathway may play a role in primary microcephaly. Molecular Genetics and Genomics, 292(2), 365–383. https://doi.org/10.1007/s00438-016-1277-x.
Sun, Y. M., Greenway, D. J., Johnson, R., Street, M., Belyaev, N. D., Deuchars, J., et al. (2005). Distinct profiles of REST interactions with its target genes at different stages of neuronal development. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E05-07-0687.
Tadesse, S., Yu, M., Kumarasiri, M., Le, B. T., & Wang, S. (2015). Targeting CDK6 in cancer: State of the art and new insights. Cell Cycle, 14(20), 3220–3230.
Tan, C. A., del Gaudio, D., Dempsey, M. A., Arndt, K., Botes, S., Reeder, A., et al. (2014). Analysis of ASPM in an ethnically diverse cohort of 400 patient samples: Perspectives of the molecular diagnostic laboratory. Clinical Genetics. https://doi.org/10.1111/cge.12172.
Tang, C. J. C., Fu, R. H., Wu, K. S., Hsu, W. B., & Tang, T. K. (2009). CPAP is a cell-cycle regulated protein that controls centriole length. Nature Cell Biology. https://doi.org/10.1038/ncb1889.
Tang, C. J. C., Lin, S. Y., Hsu, W. B., Lin, Y. N., Wu, C. T., Lin, Y. C., et al. (2011). The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO Journal. https://doi.org/10.1038/emboj.2011.378.
Taveras, C., Liu, C., & Mao, Y. (2019). A tension-independent mechanism reduces aurora B-mediated phosphorylation upon microtubule capture by CENP-E at the kinetochore. Cell Cycle. https://doi.org/10.1080/15384101.2019.1617615.
Tervasmäki, A., Mantere, T., Eshraghi, L., Laurila, N., Tuppurainen, H., Ronkainen, V. P., et al. (2019). Tumor suppressor MCPH1 regulates gene expression profiles related to malignant conversion and chromosomal assembly. International Journal of Cancer, 145, 2070–2081. https://doi.org/10.1002/ijc.32234.
Thériault, B. L., Pajovic, S., Bernardini, M. Q., Shaw, P. A., & Gallie, B. L. (2012). Kinesin family member 14: An independent prognostic marker and potential therapeutic target for ovarian cancer. International Journal of Cancer. https://doi.org/10.1002/ijc.26189.
Trimborn, M., Bell, S. M., Felix, C., Rashid, Y., Jafri, H., Griffiths, P. D., et al. (2004). Mutations in microcephalin cause aberrant regulation of chromosome condensation. American Journal of Human Genetics. https://doi.org/10.1086/422855.
Trimborn, M., Ghani, M., Walther, D. J., Dopatka, M., Dutrannoy, V., Busche, A., et al. (2010). Establishment of a mouse model with misregulated chromosome condensation due to defective Mcph1 function. PLoS ONE, 5(2), e9242. https://doi.org/10.1371/journal.pone.0009242.
Trimborn, M., Schindler, D., Neitzel, H., & Hirano, T. (2006). Misregulated chromosome condensation in MCPH1 primary microcephaly is mediated by condensin II. Cell Cycle. https://doi.org/10.4161/cc.5.3.2412.
Trynka, G., Hunt, K. A., Bockett, N. A., Romanos, J., Mistry, V., Szperl, A., et al. (2011). Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nature Genetics. https://doi.org/10.1038/ng.998.
Varmark, H., Llamazares, S., Rebollo, E., Lange, B., Reina, J., Schwarz, H., et al. (2007). Asterless Is a Centriolar Protein Required for Centrosome Function and Embryo Development in Drosophila. Current Biology. https://doi.org/10.1016/j.cub.2007.09.031.
Vaughan, K. T. (2005). TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. Journal of Cell Biology, 171(2), 197–200.
Venkatesh, T., Nagashri, M. N., Swamy, S. S., Mohiyuddin, S. M. A., Gopinath, K. S., & Kumar, A. (2013). Primary microcephaly gene MCPH1 shows signatures of tumor suppressors and is regulated by miR-27a in oral squamous cell carcinoma. PLoS ONE. https://doi.org/10.1371/journal.pone.0054643.
Venkatesh, T., & Suresh, P. S. (2014). Emerging roles of MCPH1: Expedition from primary microcephaly to cancer. European Journal of Cell Biology, 93, 98–105.
Verhey, K. J., Kaul, N., & Soppina, V. (2011). Kinesin assembly and movement in cells. Annual Review of Biophysics. https://doi.org/10.1146/annurev-biophys-042910-155310.
Wang, G. G., Allis, C. D., & Chi, P. (2007). Chromatin remodeling and cancer, part I: Covalent histone modifications. Trends in Molecular Medicine, 13(9), 363–372.
Wang, H., Wang, L., Erdjument-Bromage, H., Vidal, M., Tempst, P., Jones, R. S., et al. (2004). Role of histone H2A ubiquitination in polycomb silencing. Nature. https://doi.org/10.1038/nature02985.
Wang, J., Zhang, Y., Dou, Z., Jiang, H., Wang, Y., Gao, X., et al. (2019). Knockdown of STIL suppresses the progression of gastric cancer by down-regulating the IGF-1/PI3K/AKT pathway. Journal of Cellular and Molecular Medicine, 23, 5566–5575. https://doi.org/10.1111/jcmm.14440.
Wang, Q., Wang, L., Li, D., Deng, J., Zhao, Z., He, S., et al. (2013a). Kinesin family member 14 is a candidate prognostic marker for outcome of glioma patients. Cancer Epidemiology. https://doi.org/10.1016/j.canep.2012.08.011.
Wang, W. Y., Hsu, C. C., Wang, T. Y., Li, C. R., Hou, Y. C., Chu, J. M., et al. (2013b). A gene expression signature of epithelial tubulogenesis and a role for ASPM in pancreatic tumor progression. Gastroenterology. https://doi.org/10.1053/j.gastro.2013.07.040.
Wang, X., Tsai, J. W., Imai, J. H., Lian, W. N., Vallee, R. B., & Shi, S. H. (2009). Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature, 461(7266), 947–955. https://doi.org/10.1038/nature08435.
Wang, Y., Chai, Z., Wang, M., Jin, Y., Yang, A., & Li, M. (2018). COPB2 suppresses cell proliferation and induces cell cycle arrest in human colon cancer by regulating cell cycle-related proteins. Experimental and Therapeutic Medicine, 15, 777–784. https://doi.org/10.3892/etm.2017.5506.
Watanabe, S., De Zan, T., Ishizaki, T., & Narumiya, S. (2013). Citron kinase mediates transition from constriction to abscission through its coiled-coil domain. Journal of Cell Science. https://doi.org/10.1242/jcs.116608.
Waters, M. G., Serafini, T., & Rothman, J. E. (1991). 'Coatomer': A cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. https://doi.org/10.1038/349248a0.
Wei, Z., Kim, T.-S., Ahn, J. I., Meng, L., Chen, Y., Ryu, E. K., et al. (2020). Requirement of the Cep57-Cep63 interaction for proper Cep152 recruitment and centriole duplication. Molecular and Cellular Biology. https://doi.org/10.1128/MCB.00535-19.
Weijia, L., Ma, S., Bai, X., Pan, W., Ai, L., & Tan, W. (2020). Long noncoding RNA WDFY3-AS2 suppresses tumor progression by acting as a competing endogenous RNA of microRNA-18a in ovarian cancer. Journal of Cellular Physiology. https://doi.org/10.1002/JCP.29028.
Wood, A. J., Severson, A. F., & Meyer, B. J. (2010). Condensin and cohesin complexity: The expanding repertoire of functions. Nature Reviews Genetics, 11(6), 391–404.
Wood, K. W., Sakowicz, R., Goldstein, L. S. B., & Cleveland, D. W. (1997). CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. https://doi.org/10.1016/S0092-8674(00)80419-5.
Woods, C. G. (2004). Human microcephaly. Current Opinion in Neurobiology, 14(1), 112–117.
Wu, X., Johansen, J. V., & Helin, K. (2013). Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Molecular Cell. https://doi.org/10.1016/j.molcel.2013.01.016.
Wu, X., Liu, W., Liu, X., Ai, Q., & Yu, J. (2018). Overexpression of MCPH1 inhibits the migration and invasion of lung cancer cells. OncoTargets and Therapy, 11, 3111–3117. https://doi.org/10.2147/OTT.S156102.
Wu, X., Xiao, Y., Yan, W., Ji, Z., & Zheng, G. (2019). The human oncogene SCL/TAL1 interrupting locus (STIL) promotes tumor growth through MAPK/ERK, PI3K/Akt and AMPK pathways in prostate cancer. Gene. https://doi.org/10.1016/j.gene.2018.11.048.
Wu, Z., Zhu, X., Xu, W., Zhang, Y., Chen, L., Qiu, F., et al. (2017). Up-regulation of CIT promotes the growth of colon cancer cells. Oncotarget. https://doi.org/10.18632/oncotarget.18615.
Xu, D., Yao, M., Wang, Y., Yuan, L., Hoeck, J. D., Yu, J., et al. (2018a). MEKK3 coordinates with FBW7 to regulate WDR62 stability and neurogenesis. PLoS Biology, 16(12), e2006613. https://doi.org/10.1371/journal.pbio.2006613.
Xu, D., Yao, M. H., Wang, Y. Q., Yuan, L., Hoeck, J. D., Yu, J. W., et al. (2018b). MEKK3 coordinates with FBW7 to regulate WDR62 stability and neurogenesis. PLoS Biology, 16(12), e2006613. https://doi.org/10.1371/journal.pbio.2006613.
Xu, H., Choe, C., Shin, S. H., Park, S. W., Kim, H. S., Jung, S. H., et al. (2014). Silencing of KIF14 interferes with cell cycle progression and cytokinesis by blocking the p27Kip1 ubiquitination pathway in hepatocellular carcinoma. Experimental and Molecular Medicine. https://doi.org/10.1038/emm.2014.23.
Xu, X., Lee, J., & Stern, D. F. (2004). Microcephalin is a DNA damage response protein involved in regulation of CHK1 and BRCA1. Journal of Biological Chemistry, 279, 34091–34094. https://doi.org/10.1074/jbc.C400139200.
Xu, Z., Zhang, Q. I., Luh, F., Jin, B., & Liu, X. (2019). Overexpression of the ASPM gene is associated with aggressiveness and poor outcome in bladder cancer. Oncology Letters, 17, 1865–1876. https://doi.org/10.3892/ol.2018.9762.
Yajing, Z., Liu, F., Zhang, C., Ren, M., Kuang, M., Xiao, T., et al. (2020). Non-SMC condensin I complex subunit D2 is a prognostic factor in triple-negative breast cancer for the ability to promote cell cycle and enhance invasion. The American Journal of Pathology. https://doi.org/10.1016/J.AJPATH.2019.09.014.
Yamamoto, S., Jaiswal, M., Charng, W. L., Gambin, T., Karaca, E., Mirzaa, G., et al. (2014). A Drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. https://doi.org/10.1016/j.cell.2014.09.002.
Yamashita, D., Shintomi, K., Ono, T., Gavvovidis, I., Schindler, D., Neitzel, H., et al. (2011). MCPH1 regulates chromosome condensation and shaping as a composite modulator of condensin II. Journal of Cell Biology. https://doi.org/10.1083/jcb.201106141.
Yang, Y. J., Baltus, A. E., Mathew, R. S., Murphy, E. A., Evrony, G. D., Gonzalez, D. M., et al. (2012). Microcephaly gene links trithorax and REST/NRSF to control neural stem cell proliferation and differentiation. Cell, 151, 1097–1112. https://doi.org/10.1016/j.cell.2012.10.043.
Yao, X., Abrieu, A., Zheng, Y., Sullivan, K. F., & Cleveland, D. W. (2000). CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nature Cell Biology. https://doi.org/10.1038/35019518.
Yen, C. J., Yang, S. T., Chen, R. Y., Huang, W., Chayama, K., Lee, M. H., et al. (2019). Hepatitis B virus X protein (HBx) enhances centrosomal P4.1-associated protein (CPAP) expression to promote hepatocarcinogenesis. Journal of Biomedical Science. https://doi.org/10.1186/s12929-019-0534-9.
Yin, L., Jiang, L. P., Shen, Q. S., Xiong, Q. X., Zhuo, X., Zhang, L. L., et al. (2017). NCAPH plays important roles in human colon cancer. Cell Death and Disease. https://doi.org/10.1038/cddis.2017.88.
Yu, T. W., Mochida, G. H., Tischfield, D. J., Sgaier, S. K., Flores-Sarnat, L., Sergi, C. M., et al. (2010). Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nature Genetics. https://doi.org/10.1038/ng.683.
Zaqout, S., Bessa, P., Krämer, N., Stoltenburg-Didinger, G., & Kaindl, A. M. (2017). CDK5RAP2 is required to maintain the germ cell pool during embryonic development. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2017.01.002.
Zaqout, S., Ravindran, E., Stoltenburg-Didinger, G., & Kaindl, A. M. (2020). Congenital microcephaly-linked CDK5RAP2 affects eye development. Annals of Human Genetics. https://doi.org/10.1111/ahg.12343.
Zeng, S., Tao, Y., Huang, J., Zhang, S., Shen, L., Yang, H., et al. (2013). WD40 repeat-containing 62 overexpression as a novel indicator of poor prognosis for human gastric cancer. European Journal of Cancer. https://doi.org/10.1016/j.ejca.2013.07.015.
Zhang, J., Wu, X. B., Fan, J. J., Mai, L., Cai, W., Li, D., et al. (2013a). MCPH1 protein expression in normal and neoplastic lung tissues. Asian Pacific Journal of Cancer Prevention. https://doi.org/10.7314/APJCP.2013.14.12.7295.
Zhang, X., Liu, D., Lv, S., Wang, H., Zhong, X., Liu, B., et al. (2009). CDK5RAP2 is required for spindle checkpoint function. Cell Cycle. https://doi.org/10.4161/cc.8.8.8205.
Zhang, Y., Tian, Y., Yu, J. J., He, J., Luo, J., Zhang, S., et al. (2013b). Overexpression of WDR62 is associated with centrosome amplification in human ovarian cancer. Journal of Ovarian Research. https://doi.org/10.1186/1757-2215-6-55.
Zhang, Y., Yuan, Y., Liang, P., Zhang, Z., Guo, X., Xia, L., et al. (2017). Overexpression of a novel candidate oncogene KIF14 correlates with tumor progression and poor prognosis in prostate cancer. Oncotarget, 8, 45459–45469. https://doi.org/10.18632/oncotarget.17564.
Zheng, X., Ramani, A., Soni, K., Gottardo, M., Zheng, S., Ming Gooi, L., et al. (2016). Molecular basis for CPAP-tubulin interaction in controlling centriolar and ciliary length. Nature Communications. https://doi.org/10.1038/ncomms11874.
Zhong, W., & Chia, W. (2008). Neurogenesis and asymmetric cell division. Current Opinion in Neurobiology, 18(1), 4–11. https://doi.org/10.1016/j.conb.2008.05.002.
Zhou, X., Zhi, Y., Yu, J., & Xu, D. (2020). The Yin and Yang of autosomal recessive primary microcephaly genes: Insights from neurogenesis and carcinogenesis. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms21051691.
Zhou, Z. W., Tapias, A., Bruhn, C., Gruber, R., Sukchev, M., & Wang, Z. Q. (2013). DNA damage response in microcephaly development of MCPH1 mouse model. DNA Repair. https://doi.org/10.1016/j.dnarep.2013.04.017.
Zs, L., Liu, C. H., Liu, Z., Zhu, C. L., & Huang, Q. (2018). Downregulation of COPB2 by RNAi inhibits growth of human cholangiocellular carcinoma cells. European Review for Medical and Pharmacological Sciences. https://doi.org/10.26355/EURREV_201802_14380.
Zuccolo, M., Alves, A., Galy, V., Bolhy, S., Formstecher, E., Racine, V., et al. (2007). The human Nup107-160 nuclear pore subcomplex contributes to proper kinetochore functions. EMBO Journal. https://doi.org/10.1038/sj.emboj.7601642.
Acknowledgements
The authors would like to thank members of the Xu lab for their insightful discussion and help. This work was supported by the National Natural Science Foundation of China (Grants 31761133012, 31530016, and 31800683), the National Basic Research Program of China (Grant 2015CB910601), the Natural Science Foundation of Guangdong (Grant 2018A030310641), the Shenzhen Science and Technology Innovation Commission (Grants JCYJ20180507182213033, JCYJ20170412113009742 and JCYJ20170817095413908) and the startup fund for new faculty members of Shenzhen University (Grant 2018013).
Author information
Shibin Xu and Xingxuan Wu contributed equally to this review.
Affiliations
Guangdong Key Laboratory for Genome Stability and Disease Prevention, Carson International Cancer Center, Marshall Laboratory of Biomedical Engineering, Shenzhen University School of Medicine, Shenzhen, 518055, Guangdong, China
Shibin Xu, Xingxuan Wu, Bin Peng & Xingzhi Xu
College of Life Sciences, Capital Normal University, Beijing, 100083, China
Shibin Xu
Department of Chemistry, Capital Normal University, Beijing, 100083, China
Sheng-Li Cao
Corresponding authors
Correspondence to Bin Peng or Sheng-Li Cao or Xingzhi Xu.
Rights and permissions
About this article
Cite this article
Xu, S., Wu, X., Peng, B. et al. Primary microcephaly with an unstable genome. GENOME INSTAB. DIS. 1, 235–264 (2020). https://doi.org/10.1007/s42764-020-00020-z
Received29 April 2020
Revised24 June 2020
Accepted30 July 20
Published02 September 2020
Issue DateSeptember 2020
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkKeywords
Microcephaly
Centrosome
Cell cycle
Brain development
Neurogenesis
Cell division








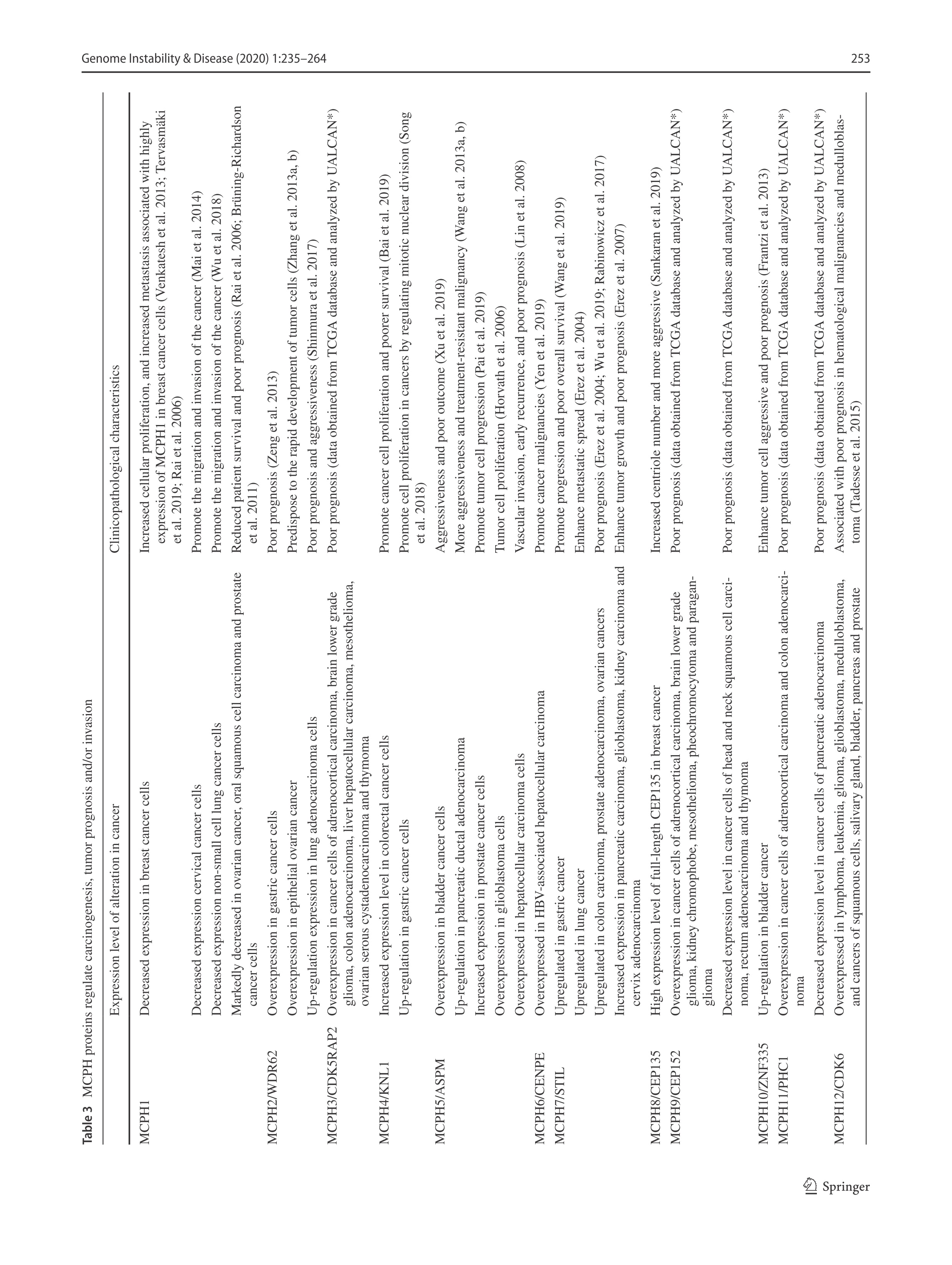
用户登录
还没有账号?
立即注册