Proteome profiling of phosphatidylinositol-5-phosphate 4-kinase type 2A and 2B knockdown cells identify modifications in key regulators involved in cell homeostasis and genome integrity
Original Research Paper
Poorwa Awasthi, Ankur Kumar Srivastava, Vipin Kumar Yadav, Radhika Singh, Smriti Singh Yadav, Gururaj Rao Kidiyoor & Amit Kumar
Genome Instability & Disease (2022)
Abstract
Phosphatidylinositol-5-phosphate 4-kinases (PIP4K) are multiple cellular process regulatory lipid kinases. PIP4K2A and PIP4K2B are overexpressed in cancer. The recently reported kinase-independent role of PIP4K2A and PIP4K2B underscore the complexity of the underlying molecular changes and mechanisms involved. Here, we show proteome analysis of PIP4K2A, PIP4K2B and PIP4K2A/2B co-depleted osteosarcoma cells to reflect changes in protein expression and their post-translational modifications. PIP4K2A depletion mainly affects ribosome assembly, translation and cell proliferation. Proteins of the apoptotic process, DNA repair, and cell division are mainly affected in PIP4K2B knockdown cells. PIP4K2A/2B co-depletion affects proteins regulating vesicle transport, cell motility, RNA splicing, and cell division. PIP4K depletion also affects post-translational modifications (phosphorylation, acetylation) of proteins involved mainly in nucleosome organization, mRNA splicing, and DNA replication. In addition, we observed PIP4K2A and PIP4K2B overexpression in cells harbouring K-Ras G12V or G12D mutations. Collectively, our results show that single or co-depletion of the PIP4K isoforms regulate proteins participating in metabolism and maintenance of genome integrity. Given that PIP4K2A and PIP4K2B alter proteins related to genome instability and their role in cancer, these enzymes could be promising therapeutic targets for cells experiencing genotoxic stress conditions.
Introduction
Phosphoinositide signalling regulates cellular processes in stress and physiological conditions (Fiume et al., 2019). Each family of phosphoinositide kinases and phosphatases has specific regulatory roles depending on the type of cell stress or growth stimulation (b; Chen, Liu, et al., 2020; Choi et al., 2019). The phosphatidylinositol-5-phosphate 4-kinase (PIP4K) family includes PIP4K2A, PIP4K2B and PIP4K2C isoforms, which phosphorylate phosphatidylinositol-5-phosphate on the fourth position of the inositol ring and catalyse PtdIns(4,5)P2 formation (Fiume et al., 2015; Rameh et al., 1997). These enzymes are central to cellular processes such as growth and development, metabolism, and tumour growth (Raghu, 2021). PIP4K2A knockdown showed selective killing of tumour cells, sparing normal hematopoietic stem and progenitor cells (Jude et al., 2015). It is also essential for myeloid malignancies. On the other hand, PIP4K2B was found to have regulatory roles in myogenic differentiation (Stijf-Bultsma et al., 2015), the cellular response to ROS (Jones et al., 2006), and sensing cellular GTP concentration (Sumita et al., 2016) in vitamin D3-induced CDH1 transcription (Kouchi et al., 2011). PIP4K2B reduced expression is associated with poor prognosis in breast cancers (Keune et al., 2013). In addition, studies related to PIP4K2A and PIP4K2B expression levels in different disease conditions found PIP4K2B overexpression in patients responsive to neoadjuvant chemotherapy in rectal cancers (Chauvin et al., 2018).
PIP4K2A and PIP4K2B double knockout in mice and in Caenorhabditis elegans leads to defects in autophagy, pointing to the ability of multicellular organisms to survive in starvation conditions (Lundquist et al., 2018). PIP4K2A and PIP4K2B double knockout also modulates insulin sensitivity (Bulley et al., 2016; Sharma et al., 2019; Wang et al., 2019), and has a synthetic lethality role in Ras-activated cancers (Kitagawa et al., 2017). Targeting PIP4K2A and PIP4K2B in p53 null mice showed reduced tumour growth (Emerling et al., 2013), suggesting a genetic interaction between p53 and PIP4K2A/PIP4K2B. These studies highlighted the potential impact of PIP4K2A and PIP4K2B in various disease pathologies, indicating the role of PIP4K2A and PIP4K2B in cell cycle regulation, proliferation and genome integrity mechanisms.
A major limitation towards understanding of how these kinases promote systemic cellular responses and safeguard genomic integrity is posed by the absence of comprehensive characterization of their downstream target proteins. To identify the potential effectors downstream of these kinases, we depleted PIP4K2A, PIP4K2B, or both in human osteosarcoma cells (U2OS), followed by large-scale proteomics analysis. Using an LC–MS/MS approach, we report that the downregulation PIP4K2A, PIP4K2B, or both is correlated with mis-regulation of proteins involved in cellular processes that impair genome instability. The analysis further revealed impact on a cluster of proteins (including post-translational modifications) involved in genome integrity maintenance.
Materials and methods
Cell culture and treatments
U2OS and 293 T cells were maintained in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, #12100046) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, #10270106) and 1X antibiotic–antimycotic (Thermo Fisher Scientific, #15240062) reagent in a humidified incubator with 5% CO2 at 37 °C.
Generation of lentivirus, viral transduction
293 T cells were used to generate lentivirus. Once cells reached 80–90% confluence in a 100 mm dish, they were transfected with opti-MEM, lentiviral vector, accessory plasmids VSVG, Rev, and PRRE using the calcium phosphate method. Virus supernatant was harvested every 24 h post-transfection for 3 days, filtered with a 0.45 μm PES syringe filter, and used to infect U2OS cells. Stably transfected U2OS cells were selected with 2 μg/ml puromycin for at least two passages, and 1 μg/ml puromycin (Sigma, #P7255) was used for continuous selection after each cell division. The shRNAs used for knockdown were PIP4K2A shRNA #TRCN0000006009 and PIP4K2B shRNA #TRCN0000338409.
Western blotting
Whole-cell lysate was prepared using equal numbers of cells by lysis in RIPA buffer (25 mM Tris HCl, 150 mM NaCl, 5 mM EDTA, 0.1% TX-100, 1% sodium deoxycholate, 0.1% SDS) supplemented with a protease inhibitor cocktail. After incubation on ice (20 min), lysates were cleared by centrifugation (14,000xg). The lysate protein concentration was measured by the BCA method; equal amounts (40 μg) of protein were added to Lammeli buffer and boiled (10 min, 95 °C). Proteins were separated in 10–12% SDS-PAGE gels and transferred to 0.45 μm nitrocellulose membranes at 250 mA (1 h). Membranes were blocked in 5% non-fat dry milk in TBST and incubated with primary antibody (overnight, 4 °C) on a shaker. All primary antibodies were diluted at a 1:1000 ratio for immunoblotting. HRP-conjugated secondary antibodies were used at a 1:10,000 ratio for chemiluminescent detection of primary antibody binding. Immunoblots were developed with Supersignal Femto or ECL substrates (Thermo Fisher Scientific) and imaged in a MyECL gel imager. Protein band intensity was quantified using ImageJ software. The immunoblots with unsaturated exposure were used for quantification with appropriate loading controls as standards. Data were analysed statistically in Microsoft Excel, using data from at least three independent experiments. The following primary antibodies were used in this study: PIP4K2B (CST, #9694), PIP4K2A (CST, #5527), beta-actin (CST, #3700). The following secondary antibodies were used: HRP-conjugated anti-rabbit IgG (#111-035-144) and anti-mouse IgG (#115-035-146; both from Jackson ImmunoResearch).
Real-time qPCR
Equal numbers of cells were lysed and total RNA was prepared using RNAeasy plus kit (Qiagen, #74134) following the manufacturer’s protocol. Quantity and quality of RNA was checked using Nanodrop, and integrity confirmed in agarose gel. Any traces of genomic DNA were degraded using amplification grade DNaseI (Sigma, #AMPD1) and cDNA was synthesized using a high-capacity cDNA synthesis kit (Thermo Scientific, #4368814). qRT-PCR was performed using PowerUp SYBR Green Master Mix (Thermo Scientific, #A25778), with primer sequences as follow: GAPDH(FP): TGCACCACCAACTGCTTAGC; GAPDH(RP): GGCATGGACTGTGGTCATGAG; PIP4K2A(FP): CGTAGCGCAGAAAGTGAAGC; PIP4K2A(RP): AGGCGAGTTTTCATGGCACT; PIP4K2B(FP): GTGTATTTGGGCTAGATGGGA; PIP4K2B(RP): AAGTCTCTTTCGGTGTCACAG. Data were analyzed with GraphPad Prism 7.
Label-free quantitative LC–MS/MS proteomics
Proteins isolated from whole cell lysate were acetone-precipitated, denatured with 8 M urea, treated with DTT (Thermo Scientific, #20,290) to a final concentration of 1 mM (1 h, room temperature), and iodoacetamide (Sigma, #I1149) to a final concentration 5 mM (1 h, in the dark, room temperature), then digested with trypsin (Promega, #5280) at a 1:40 ratio (16 h, 37 °C) in a thermomixer. Trypsin was then neutralized with 10 μl formic acid, samples were incubated (1 h, 4 °C), and any precipitate was cleared by centrifugation (4000xg, 10 min). The samples were lyophilized using a vacuum evaporator. The subsequent procedure was as described (see (Srivastava et al., 2020). Briefly, lyophilized samples were suspended in a 0.1% formic acid solution. Beta galactosidase digest (Sciex, #4465938) was added to each sample for normalization. A Nanoflow HPLC instrument (EASY-nLC 1200 system; Thermo Fisher Scientific) coupled to a Q-Exactive mass spectrometer with a nano-electrospray ion source was used for peptide identification. Chromatography was performed with two-column system. C18 reverse-phase column was used to separate the peptide mixtures at a flow rate of 300 nL/min on a 210 mini-gradient (0–10% B: 00 min, 10–30% B: 120 min, 30–45% B: 70 min, 45–90% B: 5 min, 90–95% B: 5 min, 95–95% B: 10 min). Peptide analysis was performed using data-dependent MS/MS acquisition with a dynamic exclusion duration of 60 ms. The maximum injection time was 100 ms and resolution was 70,000 with the automatic gain control of targets at 3 × 106 in MS1 mode. Maximum injection time was 60 ms, resolution was 17,500 and the automatic gain control target was 1 × 105 in MS2 mode. The data were processed using the Proteome Discoverer 2.5 proteomics platform (Thermo Fisher Scientific). These fragmentation spectra were matched in a UniProt Homo sapiens database with the precursor mass tolerance at 10 ppm. 1% false discovery rate (FDR) was set for peptide identification.
LC–MS/MS data analysis for functional and interaction analysis
A proteome list from each condition was prepared by selecting proteins detected in at least three technical replicates with more than two unique peptide sequences. To highlight changes in abundance of proteins between control and PIP4K-depleted cells, we measured protein levels through mass-spec and the abundance quantified in Proteome Discoverer. As the experiment was conducted in replicates, individual protein score was averaged across all replicates. Selective functional proteins whose abundance fold change in PIP4K vs wild-type (plko.puro) levels were > 2 were listed as upregulated; those whose abundance fold change in PIP4K vs control (plko.puro) levels were < 0.5 were listed as downregulated. We performed Gene Ontology (GO) analysis using DAVID (https://david.ncifcrf.gov/) (da Huang et al., 2009; Huang et al., 2009) to generate enriched terms for biological processes (GOTERM_BP) (p value with Benjamini correction < 0.05). Functional protein association networks were generated using STRING (Search Tool for the Retrieval of Interacting Genes/Proteins). Common and exclusively regulated proteins among different samples were grouped in a Venn diagram using Venny 2.1 software.
Cell survival assay
Post-stable cell line generation, effect of shRNA on cell survival. Cell death was estimated by a propidium iodide uptake assay (Thermo Scientific, #P1304MP). For this assay, 0.25 × 106 cells were plated in 60 mm petri dish and harvested 1 day after plating. Medium was also centrifuged to collect dead cells. The resultant pellet was resuspended in 500 μl PBS and propidium iodide (0.1 μg/ml) was added (2 min) before acquisition in an Attune NxT flow cytometer (Thermo Scientific).
Cell cycle assay
For the cell cycle assay, 0.25 × 106 cells were plated and harvested as above. The pellet was washed with 1X PBS, centrifuged (300 xg, 5 min), and cells were fixed in 70% cold ethanol (overnight − 20 °C). The next day, cells were collected by centrifugation (300 xg, 5 min) and washed twice with PBS (300xg, 5 min). The resulting cell pellet was resuspended in 500 μl FxCycle PI/RNase staining solution (Thermo Fisher #F10797) and processed for cell cycle analysis in an Attune NxT flow cytometer.
RNA synthesis
Transcription status was determined by estimating ethynyl uridine (EU) incorporation during RNA synthesis using the Click-iT EU RNA synthesis kit (Thermo Scientific, #C10329). Briefly, 0.2 × 106 cells were seeded on glass coverslips in a six-well plate. One day after plating, UV treatment was given according to the kit protocol, except Vectashield with DAPI was used as mounting medium. Slides were stored at 4 °C until imaging. Images were acquired with a LSM880 confocal microscope (Zeiss, Germany) with a 63 × oil immersion objective. Z-stack images were taken at 0.5 μm thickness of each frame. Images were analyzed in ImageJ software.
DNA synthesis
Replication status was determined by estimating ethynyl deoxyuridine (EdU) incorporation during DNA synthesis using the Click-iT EdU DNA synthesis kit (Thermo Scientific, #A10044). Briefly, 0.2 × 106 cells were seeded on glass coverslips in a 6-well plate, incubated, UV-treated, imaged, and analyzed as above.
Results
PIP4K2A knockdown alters cellular proteome and thus affects ribosome assembly, proliferation, and genome integrity
PIP4K2A was depleted in U2OS cells using PIP4K2A-specific shRNA (Emerling et al., 2013), and efficient PIP4K2A depletion was confirmed by Western blotting (Fig. 1a) and real-time qPCR (Fig. 1b). The control and PIP4K2A-depleted cells were lysed and processed for protein isolation, followed by trypsin digestion, and samples were prepared for nLC/MS–MS injection. The nLC/MS–MS data were obtained from triplicates, processed to eliminate contaminants, and analyzed using Proteome Discoverer software (scheme, Fig. 1c). The proteins identified were further filtered to determine statistical significance from triplicate samples (log2FC > 1 and p value < 0.05). The significant unique proteins from controls (wild-type cells) and PIP4K2A-depleted samples were compared; 127 up- and 27 downregulated proteins were unique to PIP4K2A-depleted cells (Fig. 1d, Table S1). The majority of these upregulated proteins were involved in negative regulation of ribosome assembly, apoptosis, translation, and cell proliferation (Fig. 1e). Additionally, proteins involved in the regulation of immune response, glucose starvation were also identified (Fig. 1e), consistent with known PIP4K2A function in autophagy and immune response (Jude et al., 2014; Lundquist et al., 2018). The observed accumulation of ribosome and metabolic proteins could be due to impaired recycling of these proteins through autophagy. We examined the autophagy status in PIP4K2A-depleted cells by immunoblotting Lc3B, key components of macroautophagy by immunoblotting Lc3B, p62 and measured the autophagy flux (Fig. 1f). We inhibited the lysosomal degradation using bafilomycin A1 (Baf A1) and analysed Lc3B and p62 accumulation (Rubinsztein et al., 2009). An increase in Lc3B levels with reduced p62 were observed in PIP4K2A-depleted cells compared to control cells (plko.puro), however, levels of both the proteins were further increased post-Baf A1 treatment in PIP4K2A-depleted cells (Fig. 1f) indicating an increased autophagy flux upon PIP4K2A depletion.
Fig. 1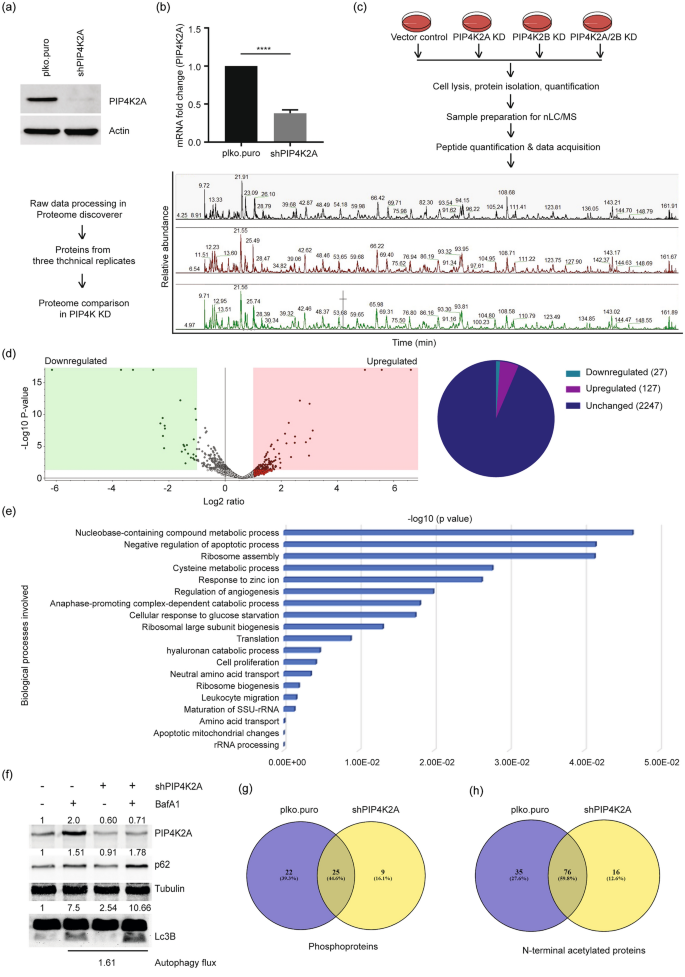
PIP4K2A knockdown alters the cellular proteome impacting genome integrity. a, b PIP4K2A was depleted in U2OS cells using shRNA. Knockdown efficiency was determined by immunoblotting using specific antibody (a) and real-time qPCR (b). PIP4K2A RNA fold change was normalised with GAPDH values. Significance was calculated using a t test, ****P < 0.0001. c Scheme of sample preparation for nLC/MS. Vector control (plko.puro) and PIP4K knockdown (PIP4K KD) cells were harvested, lysed, proteins were isolated and quantified. An equal quantity of proteins was denatured, alkylated, and digested with mass-spec grade trypsin. Salts were removed from peptide samples; an equal quantity of peptide sample was injected into nLC/MS for sample acquisition. Represented spectra show base peaks from three technical replicates analysed in Xcalibur. d Volcano plot showing up- and downregulated proteins in PIP4K2A-depleted cells compared with that of proficient cells (left panel). The volcano plot is plotted between log2 of the fold changes on the x-axis versus the − log10 p value on the y-axis. Boxes at the left represent downregulated proteins, boxes on the right represent upregulated proteins with p value < 0.05 and log2 fold change equal to one. Data analyzed by Proteome Discoverer version 2.5 (Thermo Fisher). Pie diagram of proteins found in the volcano plot (right). e Gene Ontology (GO): biological process of all upregulated proteins in the PIP4K2A-depleted cell proteome. f Immunoblot analysis for the indicated antibodies in wild type and PIP4K2A-depleted U2OS cells. Tubulin was used as a loading control and intensities are mentioned above the respective blots. Autophagy flux was determined by difference between the increased Lc3B intensity caused by Baf A1 treatment in the presence and absence of PIP4K2A. g, h Venn diagram represents the number of common or exclusive phosphoproteins (g) and Nt-acetylated proteins (h) in the presence (plko.puro) or absence (shPIP4K2A) of PIP4K2A
Full size imageWe identified WD repeat domain 43 (WDR43), WD repeat domain 75 (WDR75), Sp100, Aurora kinase A (AURKA), Homeodomain-interacting protein kinase 2 (HIPK2), DDX51, HMGN1, and ERCC2 were specifically enriched in PIP4K2A-depleted cells. These proteins regulate genome integrity mechanisms by modulating cell cycle, cell division, DNA damage response, p53-dependent gene expression, and R-loop formation (Bi et al., 2019; Moller et al., 2003; Rochman et al., 2011; Sergeeva & Zatsepin, 2021; Sokolova et al., 2017). The upregulation of these proteins is associated with different types of cancer; WDR43 and WDR75 are upregulated in colorectal cancer (CRC) (Sun & Qian, 2018), AURKA expression is altered in B cell lymphoma, prostate, and breast cancers (Dauch et al., 2016; Richards et al., 2016; Zheng et al., 2016), and HIPK2 overexpression is associated with metastasis (Feng et al., 2017). DDX51 is upregulated in non-small cell lung cancer (NSCLC) and breast cancer (Sergeeva & Zatsepin, 2021), ERCC2 correlates positively with tumour grade and stage in head and neck cancer patients, and in colorectal cancer (Liu et al., 2018; Ni et al., 2014; Zafeer et al., 2016).
The 27 downregulated proteins unique to PIP4K2A-depleted cells included SPARC (extracellular matrix protein), DSG1 (desmosomal protein), SCAF1 (splicing factor), PLCB3 (calmodulin-binding protein), PALLD, EHD2 (cell adhesion molecule-binding protein), PEX14 (receptor-binding proteins), and SLC35B2 (transporter protein) (Table 1). Of these, SPARC was identified as a tumour suppressor in urothelial cancer (Said, 2016), and PALLD mutated variants are reported in pancreatic cancer (Pogue-Geile et al., 2006). Taking these data together, PIP4K2A has regulatory role on expression of proteins implicated in uncontrolled proliferations and tumorigenesis.
Table 1 Downregulated proteins in PIP4K2A-depleted cell proteomeFull size tableSince post-translational modifications including phosphorylation and acetylation are major contributors to mitigating the DNA damage response (b; Chen, Liu, et al., 2020; Dantuma & van Attikum, 2016) and genome instability, we analysed the proteomics data for the phosphorylation status of proteins identified in controls and PIP4K2A-depleted cells. Controls show 47 unique phosphoproteins, whereas 34 phosphoproteins were unique to PIP4K2A depletion (Fig. 1g, Table S2). In comparison to controls, PIP4K2A-depleted cells lost 22 and gained 9 phosphoproteins (Table 2). Acetylation analysis of the proteins identified showed 111 and 92N-terminal acetylated (Nt-acetylated) proteins in controls and PIP4K2A-depleted cells, respectively; 35 acetylation events were control-specific and 16 were PIP4K2A-specific (Fig. 1h, Table S3). We observed that 16 acetylated proteins, such as peptidyl-prolyl isomerase (FKBP4), replication complex protein (GINS2), pre-mRNA splicing complex protein (HNRNPF, LUC7L3), and mitotic spindle assembly checkpoint protein (MALD1L1) indicating a probable role in cell proliferation. Collectively, these results suggest a potential regulatory function of PIP4K2A in controlling cellular processes such as replication, splicing, proliferation, and cell cycle by modulating protein levels or post-translational modifications of proteins involved that are promising therapeutic targets in cancer.
Table 2 Exclusively phosphorylated proteins in PIP4K2A-depleted cell proteomeFull size tablePIP4K2B knockdown affects cellular proteome and upregulates cell cycle and DNA repair regulatory proteins
We depleted PIP4K2B using specific shRNA, and tested PIP4K2B knockdown by Western blot using PIP4K2B-specific antibody (Fig. 2a) and by real-time qPCR to confirm PIP4K2B mRNA expression (Fig. 2b). Compared to controls, we observed upregulation of 173 and downregulation of 11 proteins in PIP4K2B-depleted cells (Fig. 2c, Table S1). Upregulated proteins belong to networks that regulate cell cycle (including AURKA, ARF6, BRCC3, GNAI1, CDC20), transcription, DNA repair (POLE, RBM14, PARP2, RRM2B, GTF2H4), and apoptotic signalling (CASP3, CAV1, DIDO1, PRKCA, BCL2L1, TP53I3) (Fig. 2d, e). In addition, proteins involved in mitotic spindle organization and transcription were enriched (POLR2D, GTF2B, GTF2E1, TUBG1, RBM14-RBM4). The downregulated proteins identified are known to participate in maintenance of chromosome integrity (RPS6KA3, FBXO3, CKS2) (Table 3).
Fig. 2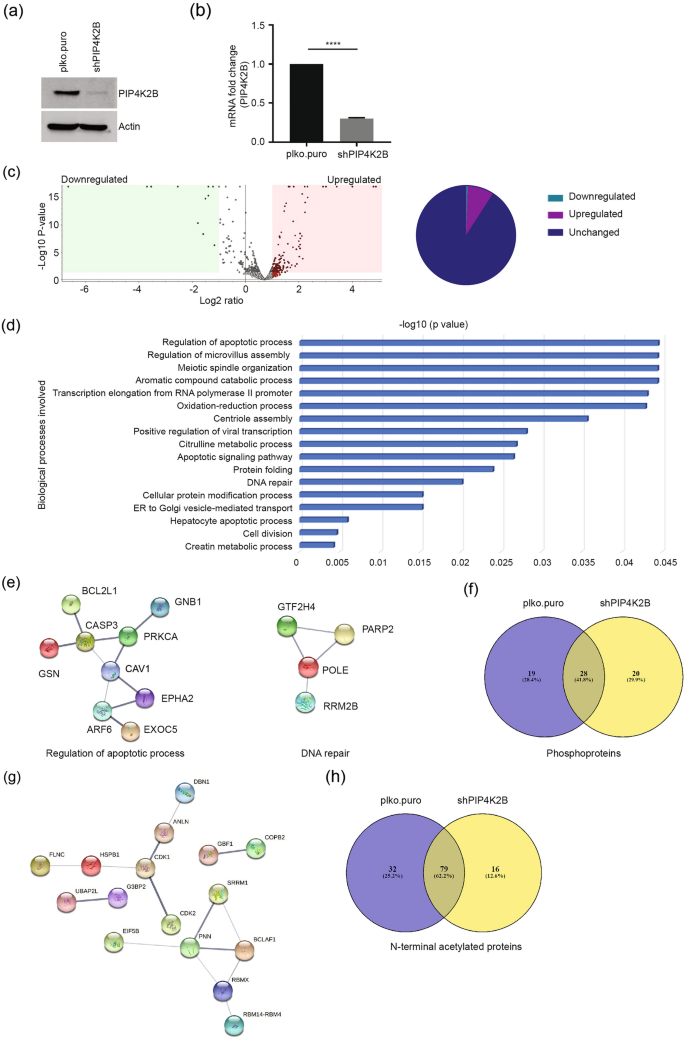
Altered cell cycle regulators in PIP4K2B knockdown cells. a, b PIP4K2B was depleted in U2OS cells using shRNA. Knockdown efficiency was determined by immunoblotting (a) and real-time qPCR (b). Fold change in PIP4K2B RNA was normalised with GAPDH values. Significance was calculated using a t test, ****p < 0.0001. c Volcano plot showing up and downregulated proteins in PIP4K2B-depleted cells compared with that of proficient cells (upper panel). The volcano plot is plotted between log2 of the fold changes on the x-axis versus the − log10 p value on the y-axis. Boxes on the left represent downregulated proteins, boxes on the right represent upregulated proteins with a p value < 0.05 and log2 fold change equal to one. Data analyzed by Proteome Discoverer version 2.5. Pie diagram of the proteins in the volcano plot (right panel). d Gene Ontology (GO): biological process of all upregulated proteins in the PIP4K2B-depleted cell proteome. e STRING network among proteins of two biological processes from (d). Line thickness among interactions shows strength of data support with confidence score of 0.400. f Venn diagram representing common and exclusive phosphoproteins in the presence (plko.puro) or absence of PIP4K2B (shPIP4K2B). g STRING network among phosphoproteins shows proteins with a role in genome stability regulating processes. Line thickness among interactions shows strength of data support with confidence score of 0.400. h Venn diagram shows common and differential N-terminus acetylated proteins in the presence (plko.puro) or absence of PIP4K2B (shPIP4K2B)
Full size imageTable 3 Downregulated proteins in PIP4K2B-depleted cell proteomeFull size tableThe data analysis for identification of phosphoproteins revealed 47 phosphoproteins in controls and 48 phosphoproteins in PIP4K2B-depleted cells (Table S2). Of these, 20 phosphoproteins were exclusive to PIP4K2B-depleted cells (Table 4), whereas 19 were unique to controls (Fig. 2f). PIP4K2B-specific phosphoproteins are involved in splicing, RNA binding, and genome integrity maintenance (Fig. 2g), whereas phosphoproteins identified only in controls participate in nucleosome assembly (NAPL1L4), ribosome biogenesis (NOP56), RNA binding (RBM25), splicing, gene expression (SRRM2, YAP1) and translation (EEF1D). These phosphoproteome analysis results suggest additional PIP4K2B function in nucleosome and in nuclear events.
Table 4 Exclusively phosphorylated proteins in PIP4K2B-depleted cell proteomeFull size tableProtein acetylation analysis identified 95 Nt-acetylated proteins in PIP4K2B knockdown cells, and 111 Nt-acetylated proteins in controls (Fig. 2h, Table S3). Of these, 16 acetylated events were specific to PIP4K2B knockdown and 32 events were specific to controls (Fig. 2i). These results indicate the role of PIP4K2B-mediated regulatory mechanisms in protein stability, protein–protein interaction, or sub-cellular protein localization via regulation of N-terminal acetyl modification. Collectively, these results point to a strong correlation between mis-regulation of various genome stability regulatory proteins and their phosphorylation following PIP4K2B depletion. Comparison with PIP4K2A-dependent effectors showed that PIP4K2A and PIP4K2B have distinct cellular regulatory functions. PIP4K2A regulates proteins of ribosome assembly, translation, cell proliferation, and glucose metabolism, whereas PIP4K2B has a regulatory function in proteins of spindle organization, centriole assembly, apoptosis, DNA repair, and cell division.
PIP4K2A/2B co-depletion affects gene expression regulators
PIP4K2A and PIP4K2B are redundant (Bultsma et al., 2010) and participate in similar cellular processes (Fiume et al., 2015). Since we identified distinct regulatory functions for PIP4K2A and PIP4K2B, we determined the redundancy between the two. Both enzymes were co-depleted in U2OS cells, and efficient depletion of both the protein (Fig. 3a) and mRNA (Fig. 3b, c) was examined. Comparative proteome analysis between control and PIP4K2A/PIP4K2B co-depleted cells show upregulation of 13 proteins and downregulation of 211 proteins in co-depleted cells (Fig. 3d, Table S1). Nucleotide-binding proteins, mRNA stability, mRNA transcription proteins (SERBP1, CKMT1A, CKMT1B, BTAF1, USP22), and other proteins were among the upregulated proteins identified (Table 5). Many cell cycle proteins (CCNA2, POLE3, SUN2, UBE2C, NUF2, SPC25, PBK, CDK6), histone core complex proteins (HIST1H1A, HIST2H2AB), and splicing factors (SF3A2, SF3B4, PRPF4B) were among downregulated proteins in the PIP4K2A/2B-depleted cell proteome (Fig. 3e). Altered expression of these proteins is reported in many cancers (Bayard et al., 2018; Davidsson et al., 2009; Gao et al., 2014; Kim et al., 2018; Tadesse et al., 2015; Wang et al., 2017), and downregulation is linked to increased genome instability (Bellelli et al., 2018; Choi & Anders, 2014; Dai et al., 2021; Lei et al., 2012). Since PIP4K2A-depleted cells show impaired autophagy, we examined autophagy levels in PIP4K2A/B co-depleted cells as well by immunoblotting Lc3B, p62 and measured the autophagy flux in presence of Bafilomycin A1. PIP4K2B depletion alone did not affect the autophagy process in comparison to the PIP4K2A depletion effect (Fig. 3f); however, autophagy flux is increased in co-depleted cells similar to PIP4K2A-depleted cells. This suggests that the increased autophagy flux in co-depleted cells is mainly due to the absence of PIP4K2A. This is consistent with previously described role of PIP4K2A and PIP4K2B in autophagy regulation (Lundquist et al., 2018).
Fig. 3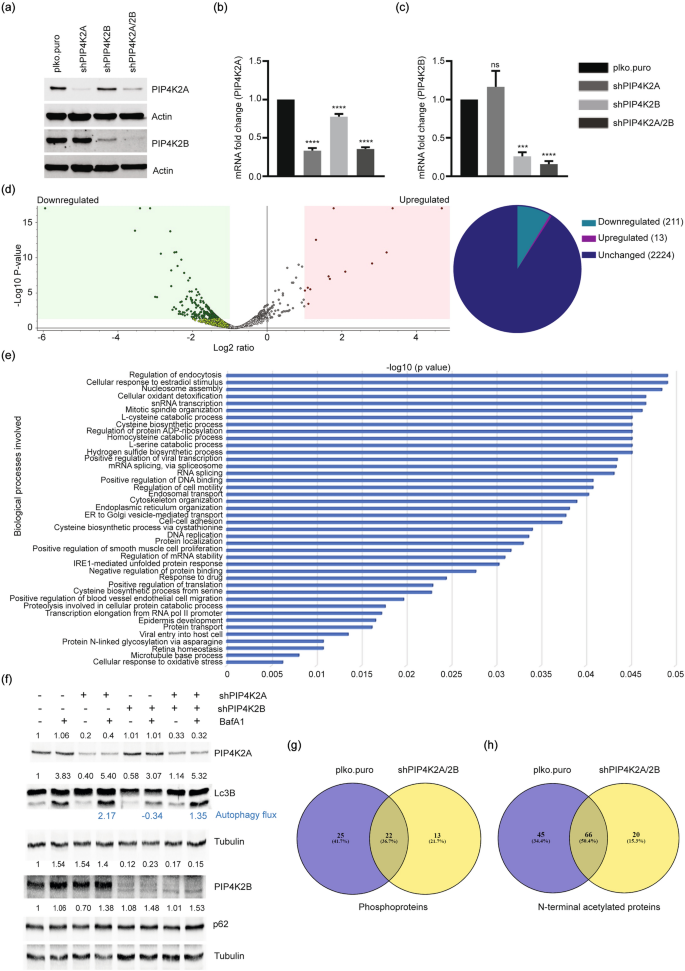
PIP4K2A/2B co-depletion affects gene expression regulators. a–c PIP4K2A/2B were depleted in U2OS cells using shRNA. Knockdown efficiency was determined by immunoblotting (a) and real-time qPCR (b, c). Fold change in PIP4K2A (b) and PIP4K2B (c) RNA was normalised with GAPDH values. Graphs were generated in GraphPad Prism 7; significance was calculated using one-way ANOVA and Dunnett’s multiple comparisons test, p (ns) = 0.1234, ***p < 0.0002, ****p < 0.0001. d Volcano plot showing up- and downregulated proteins in PIP4K2A/2B double-depleted cells compared with that of proficient cells (left panel). Volcano plot is plotted between log2 of the fold changes on the x-axis versus the − log10 p value on the y-axis. Boxes on the left represent downregulated proteins, boxes on the right represent upregulated proteins with p value < 0.05 and log2 fold change equal to 1. Data analyzed by Proteome Discoverer version 2.5. Pie diagram of the proteins found in the volcano plot (right panel). e Gene Ontology (GO): biological process of all downregulated proteins in the PIP4K2A/2B-depleted cell proteome. f Immunoblot analysis for the indicated antibodies in wild type and PIP4K2A-, PIP4K2B-, and PIP4K2A/2B-depleted U2OS cells. Tubulin was used as a loading control and fold changes are mentioned above the respective blots. Autophagy flux was determined by difference between the increased Lc3B intensity caused by Baf A1 treatment in the control, PIP4K2A, PIP4K2B or PIP4K2A/PIP4K2B co-depleted cells. g, h Venn diagram showing common or exclusive phosphoproteins (g), Nt-acetylated proteins (h) in the presence (plko.puro) or absence of both isoforms PIP4K2A/2B (shPIP4K2A/2B)
Full size imageTable 5 Upwnregulated proteins in PIP4K2A/2B-depleted cell proteomeFull size tablePhosphoprotein analysis revealed 47 phosphoproteins in controls and 35 phosphoproteins in PIP4K2A/2B co-depleted cells (Fig. 3g, Table S2). Of these, 25 phosphorylated proteins were specific to controls and 13 were PIP4K2A/2B co-depletion dependent (Fig. 3g, Table 6). Phosphorylation of DDX21 S121, RBM39 S136 and SRSF9 S211 splicing factors was lost in PIP4K2A/2B co-depleted cells. p-DDB2 (serine 26) (involved in nucleotide excision repair) and RBM25 (splicing factor) phosphorylation at S675 were not observed in PIP4K2A/2B co-depleted cells (Matsumoto et al., 2015), suggesting that PIP4K2A and 2B isoforms coordinate in phosphorylation of proteins linked to the DNA damage response, cell cycle and gene expression thereby connecting these post-translational modifications to genome integrity maintenance.
Table 6 Exclusively phosphorylated proteins in PIP4K2A/2B-depleted cell proteomeFull size tableAnalysis of acetylated proteins showed 111 acetylated proteins in controls and 86 acetylated proteins in PIP4K2A/2B co-depleted cells (Fig. 3h, Table S3), of which 20 were co-depletion specific and 45 were unique to controls (Fig. 3h). These results provide indirect evidences that PIP4K2A/2B regulates post-translational events that control protein stability, interaction, and sub-cellular localisation through N-terminal acetylation regulation.
Specific and redundant role of PIP4K2A and PIP4K2B in cellular proteome regulation
We have identified specific up- and downregulated proteins in PIP4K2A, PIP4K2B, and PIP4K2/2B double-knockdown cells with exclusive post-translational modification (phosphorylation and acetylation). We noted that some of these events were common in PIP4K2A, PIP4K2B, and PIP4K2A/2B double-knockdown cells. To analyse the effect of individual PIPKs and their physiological relevance relative to their contribution in specific cell processes, we, therefore, compared each PIP4K knockdown cell proteome. Upregulation of 101, 147, and 4 proteins were PIP4K2A, PIP4K2B, and PIP4K2A/2B co-depletion specific, respectively (Fig. 4a, Table S4). Similar comparison of downregulated proteins showed maximum downregulation in PIP4K2A/2B double-knockdown cells (194 proteins); 18 proteins were downregulated in PIP4K2A knockdown cells, whereas only 6 were specifically downregulated in PIP4K2B knockdown cells (Fig. 4b). This finding suggests that co-depletion of both PIP4K isoforms had a synergistic effect in the downregulation of a large number of proteins.
Fig. 4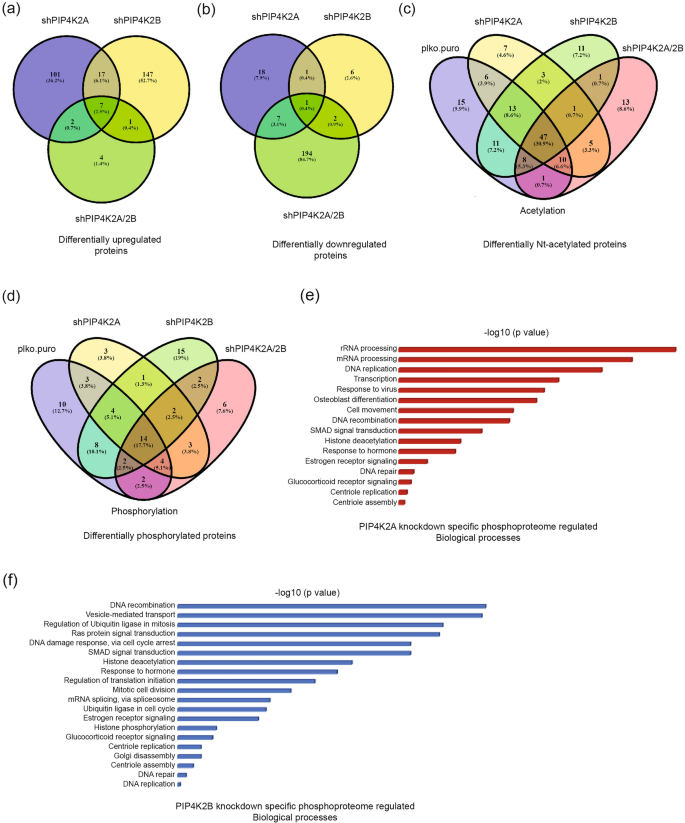
Specific and redundant roles of PIP4K2A and PIP4K2B in cellular proteome regulation. a Venn diagram showing commonly and differentially upregulated proteins in the PIP4K2A, PIP4K2B, and PIP4K2A/2B cell proteomes. b Venn diagram showing commonly and differentially downregulated proteins in the PIP4K2A, PIP4K2B, and PIP4K2A/2B cell proteomes. c Venn diagram showing commonly and exclusively N-terminal acetylated proteins in the PIP4K2A, PIP4K2B, and PIP4K2A/2B cell proteomes. d Venn diagram showing commonly and exclusively phosphorylated proteins in the PIP4K2A, PIP4K2B, and PIP4K2A/2B cell proteomes. e Phosphorylated proteins in the PIP4K2A-depleted cell proteome were analysed for involvement in different biological processes, using Database for Annotation, Visualization and Integrated Discovery (DAVID). f Phosphorylated proteins in the PIP4K2B-depleted cell proteome were analysed for involvement in different biological processes, using DAVID
Full size imageAcetylation studies in PIP4K knockdown cells identified 11, 7, 11, and 13 Nt-acetylated proteins exclusive to WT, PIP4K2A-, PIP4K2B- and PIP4K2A/2B-knockdown cells, respectively (Fig. 4c, Table S4). PIP4K2A knockdown-specific acetylated proteins included ribosomal protein (RPS18), translational protein (EIF2A), and a cytoprotective protein (HMOX1). PIP4K2B knockdown-specific acetylated proteins include some genome stability regulating proteins such as STK4 (serine/threonine-protein kinase; a stress-induced kinase) and PBK (altered expression in various cancers). BAG6 (autophagy protein), HNRNPK (splicing protein) were among other acetylated proteins found exclusively in PIP4K co-depleted cells.
Phosphoprotein studies identified 10, 3, 15 and 6 exclusive phosphorylated proteins in samples from WT, PIP4K2A-, PIP4K2B- and PIP4K2A/2B-depleted cells, respectively (Fig. 4d, Table S4). PIP4K2A knockdown-specific phosphorylated proteins were DNA damage repair protein (RPS3), splicing protein (SNRNP200), and a multifunctional protein (PAK4). PIP4K2B knockdown-specific phosphorylated proteins were strongly correlated and belong to splicing, cell cycle, and gene expression regulatory proteins. Comparative analysis of phosphorylated proteins found in PIP4K2A- and PIP4K2B-depleted cell proteomes offers insight into the role of these proteins in various cellular processes (Fig. 4e, f). Phosphorylated proteins in PIP4K2A-depleted cells had a major role in rRNA processing, mRNA processing, DNA replication, transcription, and many other processes that maintain genome stability (Fig. 4e), whereas in PIP4K2B-depleted cells, phosphorylated proteins had a more important role in recombination, vesicle-mediated transport, and ubiquitination in cell division (Fig. 4f). These results highlight PIP4K2A- and PIP4K2B-dependent events and specific function in different processes, along with synergistic regulation by both PIP4K2A and PIP4K2B (Fig. 4a, b).
We observed that PIP4K2A and PIP4K2B clearly affected proteome involved in cell cycle regulation, stress response, replication and transcription. We thus performed a related experiment to test their impact in physiological conditions, and found no alterations in cell cycle progression in any of the PIP4K-depleted cells (Fig. 5a). We assessed whether, if many stress response proteins and apoptosis-regulating proteins were upregulated in the absence of PIP4K, there could be increased apoptosis. We observed no apoptosis (Fig. 5b), which suggests a compensatory function of another PIP kinases to maintain cellular homeostasis; this is consistent with other reports (Wang et al., 2019). Furthermore, we analysed replication (Fig. 5c) and transcription (Fig. 5d), and found no alterations in PIP4K2A-, PIP4K2B- and PIP4K2A/2B-depleted cells. These results indicate that although different PIP4K isoforms regulate genome stability-related proteins, their depletion, alone or together, leads to no defect in cell cycle progression defect in cell cycle progression, proliferation, apoptosis, or transcription. This could be due to the fact that another class of phosphoinositide kinases, the phosphatidylinositol-4-phosphate 5-kinases (PIP5K) are available to assume the PIP4K function to maintain cellular homeostasis in physiological conditions (Wang et al., 2019). As the PIP5K perform other functions in genotoxic stress conditions (Choi et al., 2019), however, it would be unlikely that they compensate PIP4K function.
Fig. 5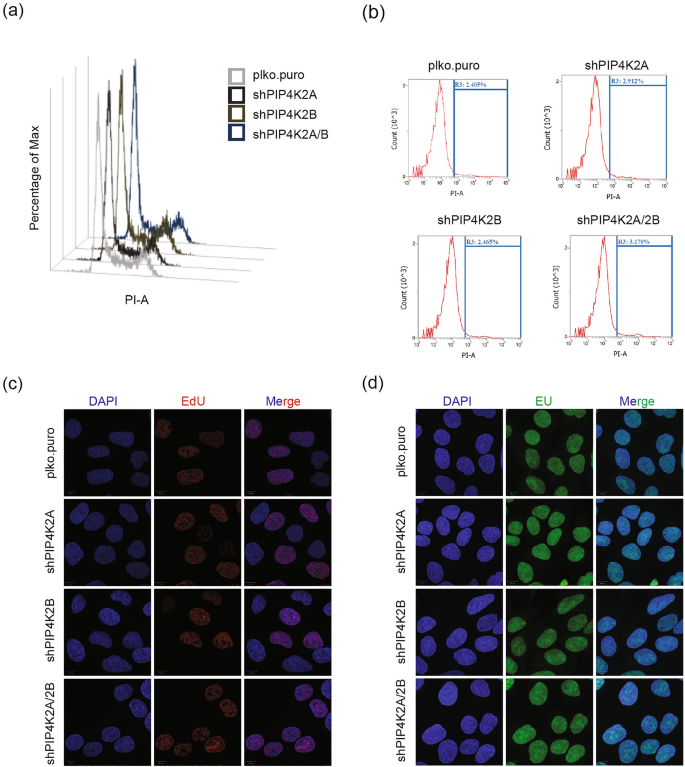
PIP4K2A and PIP4K2B knockdown-mediated genome stability in physiological conditions. a The cell cycle in plko.puro, PIP4K2A-, PIP4K2B-, and PIP4K2A/2B-depleted cells was analysed by flow cytometry. b Cell death in plko.puro, PIP4K2A-, PIP4K2B-, and PIP4K2A/2B-depleted cells was analysed by flow cytometry. Cells were harvested 24 h after cell seeding and stained with propidium iodide (PI). R3 gate represents the percentage of dead cells. c Immunofluorescence detection of ethynyl deoxyuridine (EdU). Cellular replication was confirmed by an EdU incorporation assay. DAPI was used for nuclear stain. Airy scan images of more than 100 cells were obtained at 63 × magnification and processed in ImageJ. d Immunofluorescence detection of ethynyl uridine (EU). Cellular transcription was confirmed by an EU incorporation assay. DAPI was used for nuclear stain. Airy scan images of more than 100 cells were obtained at 63 × magnification and processed in ImageJ
Full size imageSince mutation in K-Ras at G12V and G12D in different cancers is linked to poor prognosis, we tested PIP4K2A and PIP4K2B expression in K-Ras G12V mouse embryonic fibroblasts (MEF) and in K-Ras G12D-transfected CMT-93 cells by western blotting (Fig. 6a, b) and also analysed their autophagy status (Atg7, Lc3B expression) in presence or absence of Bafilomycin A (Baf A1). Protein levels of both PIP4K2A and PIP4K2B were increased in K-Ras G12V mutant MEF compared to controls. Consistent PIP4K2A and PIP4K2B overexpression paralleled elevation in Lc3B and Atg7 protein levels, suggesting hyperactivation of macroautophagy in these cells (Fig. 6a). K-Ras G-12D-overexpressing CMT-93 cells also showed PIP4K2A and PIP4K2B overexpression as well as increased Atg7 and Lc3B levels (Fig. 6b). The results suggest that both K-Ras G12V and K-Ras G12D-expressing cells have elevated PIP4K2A and PIP4K2B expression and increased macroautophagy, which could assist hyperproliferation. Further study is needed to investigate these events, which could provide mechanistic insights into the role of PIP4K in K-Ras-mediated cell transformation and tumorigenesis.
Fig. 6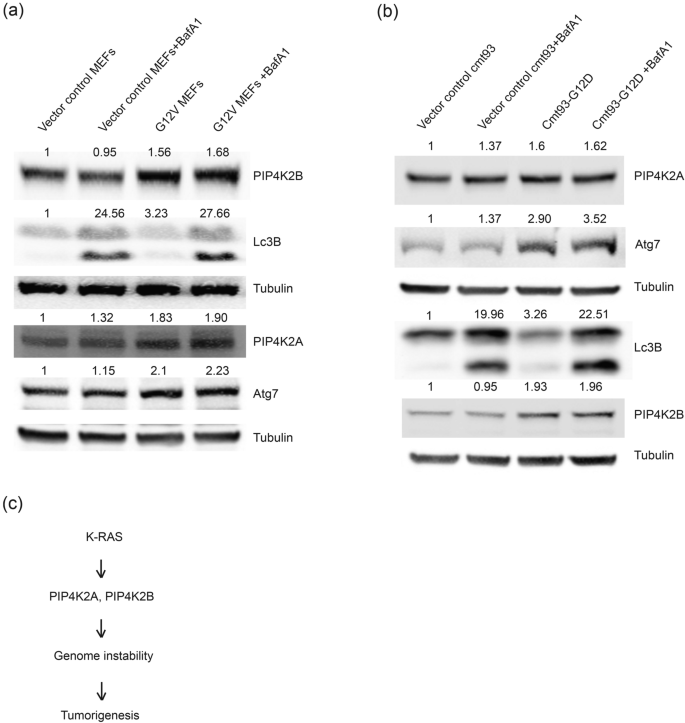
Cells expressing gain-of-function K-Ras mutants overexpress PIP4K2A and PIP4K2B with elevated autophagy. a PIP4K2A and PIP4K2B protein levels were examined by western blotting in vector control and K-Ras G12V-expressing MEF using specific antibodies, alone or with bafilomycin A1 (100 nM, 16 h). Tubulin was used as loading control and fold changes are mentioned above the respective blots. b CMT-93 cells were transfected with K-Ras G12D plasmid and PIP4K2A, PIP4K2B, Lc3B and Atg7 protein levels were examined in western blotting using indicated antibodies, alone or with BafA1 treatment (100 nM, 16 h). Tubulin was used as loading control and fold changes are mentioned above the respective blots. c Model: K-Ras gain-of-function G12V and G12D mutations induced PIP4K2A and PIP4K2B overexpression and regulate levels of proteins that control genome instability (probably through autophagy regulation) and that in turn contributed to tumour progression/tumorigenesis
Full size imageIn summary, we observed that PIP4K2A and PIP4K2B clearly affects the cellular proteome involved in cell cycle regulation, stress response, replication, and transcription. Both PIP4K2A and PIP4K2B protein levels are elevated in cells expressing the K-Ras G12V or K-Ras G12D oncogene. These results also indicate that PIP4K function may be critical in cells experiencing stress conditions (such as K-Ras oncogene expression) (Fig. 6c).
Discussion
Here, we report the effect of PIP4K isoforms on the cellular proteome as studied by mass spectrometric analysis in PIP4K2A, PIP4K2B, and PIP4K2A/2B co-depleted cells. We observed alteration in the cellular proteome regulating genomic stability. Proteins involved in autophagy, insulin signalling, and ROS signalling were also identified in this study, which were previously linked to PIP4K-mediated regulation (Emerling et al., 2013). Alteration of the above-mentioned cellular processes and their mediators has been reported in PIP4K-depleted cells (Bulley et al., 2016; Emerling et al., 2013; Lundquist et al., 2018; Stijf-Bultsma et al., 2015; Wang et al., 2019); our study is the first to use whole cell proteome analysis to identify additional regulation of genome instability regulatory proteins. Given that PIP4K2B depletion alters protein levels of different cell cycle regulatory and DNA repair proteins, the synthetic genetic interaction of PIP4K2B and tumour suppressor p53 could be explained by this finding (Emerling et al., 2013). The current study will certainly assist further proteogenomic studies in revealing the downstream mediators of PIP4K signalling that could provide promising therapeutic targets in cancer with PIP4K2A or PIP4K2B overexpression.
Emerling et al. reported that mice harbouring homozygous deletion of PIP4K2B were non-viable, which suggests PIP4K essential role in embryonic development (Emerling et al., 2013). However, the cell lines used in that study (U2OS), with PIP4K2B depletion alone or in combination with PIP4K2A, were nonetheless able to proliferate normally, consistent with recent reports (Wang et al., 2019). A synthetic genetic interaction of PIP4K2B in TP53 null mice with reduced tumour formation has been reported (Emerling et al., 2013), suggesting interplay of these genes to regulate genome integrity. Consistent with this, we found upregulation of the chromosomal organization proteins AURKA, TUBG1, CDC20 in PIP4K2B-depleted cells. Overexpression of these genes is associated with increased susceptibility to cancer and poor prognosis (Chan et al., 2017; Chen et al., 2008, 2018; Dong et al., 2019; Liu et al., 2015; Xu et al., 2017), thus reinforcing the anti-tumour potential of PIP4K inhibitors. Moreover, clusters of proteins known to function in stress response (SPARC, B2M, TRIM28), transcription (POLR2L, LSM2), and cell division (NDC80, CCNA2, SPC25) were identified in the downregulated proteome of PIP4K2A/2B co-depleted cells. Downregulation of some of these proteins decreases cancer metastasis, invasion, and cell survival (Jiang et al., 2013; Yin et al., 2010), further supporting the idea that PIP4K inhibitors might constitute promising regimens for treatment of cancers.
We also report that differential post-translational modifications (phosphorylation and N-terminal acetylation) are affected by different PIP4K isoforms, which indicates an additional layer of regulation where phosphorylation or acetylation of identified proteins was exclusive to PIP4K2A, PIP4K2B, or PIP4K2A/2B depletion. Since both the phosphorylation and acetylation processes regulate protein catalytic activity, their interaction with nucleic acid/proteins, protein half-life and intracellular localisation, our proteome data indicate a potential role for PIP4K2A and PIP4K2B in regulation of protein homeostasis as well. The proteome analysis reported here identified critical mediators of PIP4K2A or PIP4K2B signalling that could provide the basis for further studies in understanding the pathophysiological changes in PIP4K2A- or PIP4K2B-overexpressing tumours, and future studies with direct evidence showing the alteration in PTM levels of these proteins in PIP4K-depleted cells may also provide promising therapeutic targets.
Data availability
The processed data are shared on JPOST. https://repository.jpostdb.org/preview/913033704619b8641c16a8. Access key: 8637. (Announced ID; JPST001387, PXD029802). jPOSTrepo is an international standard data repository for proteomes.
References
Bayard, Q., Meunier, L., Peneau, C., Renault, V., Shinde, J., Nault, J.-C., Mami, I., Couchy, G., Amaddeo, G., Tubacher, E., Bacq, D., Meyer, V., La Bella, T., Debaillon-Vesque, A., Bioulac-Sage, P., Seror, O., Blanc, J.-F., Calderaro, J., Deleuze, J.-F., … Letouzé, E. (2018). Cyclin A2/E1 activation defines a hepatocellular carcinoma subclass with a rearrangement signature of replication stress. Nature Communications, 9(1), 5235.
Bellelli, R., Borel, V., Logan, C., Svendsen, J., Cox, D. E., Nye, E., Metcalfe, K., O’Connell, S. M., Stamp, G., Flynn, H. R., Snijders, A. P., Lassailly, F., Jackson, A., & Boulton, S. J. (2018). Polepsilon instability drives replication stress, abnormal development, and tumorigenesis. Molecular Cell, 70(4), 707-721 e707.
Bi, X., Xu, Y., Li, T., Li, X., Li, W., Shao, W., Wang, K., Zhan, G., Wu, Z., Liu, W., Lu, J. Y., Wang, L., Zhao, J., Wu, J., Na, J., Li, G., Li, P., & Shen, X. (2019). RNA targets ribogenesis factor WDR43 to chromatin for transcription and pluripotency control. Molecular Cell, 75(1), 102-116 e109.
Bulley, S. J., Droubi, A., Clarke, J. H., Anderson, K. E., Stephens, L. R., Hawkins, P. T., & Irvine, R. F. (2016). In B cells, phosphatidylinositol 5-phosphate 4-kinase-alpha synthesizes PI(4,5)P2 to impact mTORC2 and Akt signaling. Proceedings of the National Academy of Sciences, 113(38), 10571–10576.
Bultsma, Y., Keune, W. J., & Divecha, N. (2010). PIP4Kbeta interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kalpha. The Biochemical Journal, 430(2), 223–235.
Chan, S., Sridhar, P., Kirchner, R., Lock, Y. J., Herbert, Z., Buonamici, S., Smith, P., Lieberman, J., & Petrocca, F. (2017). Basal-A triple-negative breast cancer cells selectively rely on RNA splicing for survival. Molecular Cancer Therapeutics, 16(12), 2849–2861.
Chauvin, A., Wang, C. S., Geha, S., Garde-Granger, P., Mathieu, A. A., Lacasse, V., & Boisvert, F. M. (2018). The response to neoadjuvant chemoradiotherapy with 5-fluorouracil in locally advanced rectal cancer patients: a predictive proteomic signature. Clinical Proteomics, 15, 16.
Chen, C. H., Su, C. Y., Chien, C. Y., Huang, C. C., Chuang, H. C., Fang, F. M., Huang, H. Y., Chen, C. M., & Chiou, S. J. (2008). Overexpression of beta2-microglobulin is associated with poor survival in patients with oral cavity squamous cell carcinoma and contributes to oral cancer cell migration and invasion. British Journal of Cancer, 99(9), 1453–1461.
Chen, J., Chen, H., Yang, H., & Dai, H. (2018). SPC25 upregulation increases cancer stem cell properties in non-small cell lung adenocarcinoma cells and independently predicts poor survival. Biomedicine & Pharmacotherapy, 100, 233–239.
Chen, L., Liu, S., & Tao, Y. (2020). Regulating tumor suppressor genes: post-translational modifications. Signal Transduction and Targeted Therapy, 5(1), 90.
Chen, M., Wen, T., Horn, H. T., Chandrahas, V. K., Thapa, N., Choi, S., Cryns, V. L., & Anderson, R. A. (2020). The nuclear phosphoinositide response to stress. Cell Cycle, 19(3), 268–289.
Choi, S., Chen, M., Cryns, V. L., & Anderson, R. A. (2019). A nuclear phosphoinositide kinase complex regulates p53. Nature Cell Biology, 21(4), 462–475.
Choi, Y. J., & Anders, L. (2014). Signaling through cyclin D-dependent kinases. Oncogene, 33(15), 1890–1903.
da Huang, W., Sherman, B. T., & Lempicki, R. A. (2009). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research, 37(1), 1–13.
Dai, M., Boudreault, J., Wang, N., Poulet, S., Daliah, G., Yan, G., Moamer, A., Burgos, S. A., Sabri, S., Ali, S., & Lebrun, J. J. (2021). Differential regulation of cancer progression by CDK4/6 plays a central role in DNA replication and repair pathways. Cancer Research, 81(5), 1332–1346.
Dantuma, N. P., & van Attikum, H. (2016). Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO Journal, 35(1), 6–23.
Dauch, D., Rudalska, R., Cossa, G., Nault, J. C., Kang, T. W., Wuestefeld, T., Hohmeyer, A., Imbeaud, S., Yevsa, T., Hoenicke, L., Pantsar, T., Bozko, P., Malek, N. P., Longerich, T., Laufer, S., Poso, A., Zucman-Rossi, J., Eilers, M., & Zender, L. (2016). A MYC-aurora kinase A protein complex represents an actionable drug target in p53-altered liver cancer. Nature Medicine, 22(7), 744–753.
Davidsson, J., Lilljebjorn, H., Andersson, A., Veerla, S., Heldrup, J., Behrendtz, M., Fioretos, T., & Johansson, B. (2009). The DNA methylome of pediatric acute lymphoblastic leukemia. Human Molecular Genetics, 18(21), 4054–4065.
Dong, S., Huang, F., Zhang, H., & Chen, Q. (2019). Overexpression of BUB1B, CCNA2, CDC20, and CDK1 in tumor tissues predicts poor survival in pancreatic ductal adenocarcinoma. Bioscience Reports. https://doi.org/10.1042/BSR20182306
Emerling, B. M., Hurov, J. B., Poulogiannis, G., Tsukazawa, K. S., Choo-Wing, R., Wulf, G. M., Bell, E. L., Shim, H. S., Lamia, K. A., Rameh, L. E., Bellinger, G., Sasaki, A. T., Asara, J. M., Yuan, X., Bullock, A., Denicola, G. M., Song, J., Brown, V., Signoretti, S., & Cantley, L. C. (2013). Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell, 155(4), 844–857.
Feng, Y., Zhou, L., Sun, X., & Li, Q. (2017). Homeodomain-interacting protein kinase 2 (HIPK2): a promising target for anti-cancer therapies. Oncotarget, 8(12), 20452–20461.
Fiume, R., Faenza, I., Sheth, B., Poli, A., Vidalle, M. C., Mazzetti, C., Abdul, S. H., Campagnoli, F., Fabbrini, M., Kimber, S. T., Mariani, G. A., Xian, J., Marvi, M. V., Mongiorgi, S., Shah, Z., & Divecha, N. (2019). Nuclear phosphoinositides: their regulation and roles in nuclear functions. International Journal of Molecular Sciences, 20(12), 2991.
Fiume, R., Stijf-Bultsma, Y., Shah, Z. H., Keune, W. J., Jones, D. R., Jude, J. G., & Divecha, N. (2015). PIP4K and the role of nuclear phosphoinositides in tumour suppression. Biochimica Et Biophysica Acta, 1851(6), 898–910.
Gao, T., Han, Y., Yu, L., Ao, S., Li, Z., & Ji, J. (2014). CCNA2 is a prognostic biomarker for ER+ breast cancer and tamoxifen resistance. PLoS ONE, 9(3), e91771.
Huang, D. W., Sherman, B. T., & Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4(1), 44–57.
Jiang, Y., Zhu, Y., Shi, Y., He, Y., Kuang, Z., Sun, Z., & Wang, J. (2013). Downregulation of SPARC expression inhibits the invasion of human trophoblast cells in vitro. PLoS ONE, 8(7), e69079.
Jones, D. R., Bultsma, Y., Keune, W. J., Halstead, J. R., Elouarrat, D., Mohammed, S., Heck, A. J., D’Santos, C. S., & Divecha, N. (2006). Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Molecular Cell, 23(5), 685–695.
Jude, J. G., Spencer, G. J., Huang, X., Somerville, T. D. D., Jones, D. R., Divecha, N., & Somervaille, T. C. P. (2014). A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene, 34(10), 1253–1262.
Jude, J. G., Spencer, G. J., Huang, X., Somerville, T. D. D., Jones, D. R., Divecha, N., & Somervaille, T. C. P. (2015). A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene, 34(10), 1253–1262.
Keune, W. J., Sims, A. H., Jones, D. R., Bultsma, Y., Lynch, J. T., Jirstrom, K., Landberg, G., & Divecha, N. (2013). Low PIP4K2B expression in human breast tumors correlates with reduced patient survival: A role for PIP4K2B in the regulation of E-cadherin expression. Cancer Research, 73(23), 6913–6925.
Kim, J., Kim, Y., Choi, H., Kwon, A., Jekarl, D. W., Lee, S., Jang, W., Chae, H., Kim, J. R., Kim, J. M., & Kim, M. (2018). Ubiquitin C decrement plays a pivotal role in replicative senescence of bone marrow mesenchymal stromal cells. Cell Death & Disease, 9(2), 139.
Kitagawa, M., Liao, P. J., Lee, K. H., Wong, J., Shang, S. C., Minami, N., Sampetrean, O., Saya, H., Lingyun, D., Prabhu, N., Diam, G. K., Sobota, R., Larsson, A., Nordlund, P., McCormick, F., Ghosh, S., Epstein, D. M., Dymock, B. W., & Lee, S. H. (2017). Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality. Nature Communications, 8(1), 2200.
Kouchi, Z., Fujiwara, Y., Yamaguchi, H., Nakamura, Y., & Fukami, K. (2011). Phosphatidylinositol 5-phosphate 4-kinase type II beta is required for vitamin D receptor-dependent E-cadherin expression in SW480 cells. Biochemical and Biophysical Research Communications, 408(4), 523–529.
Lei, K., Zhu, X., Xu, R., Shao, C., Xu, T., Zhuang, Y., & Han, M. (2012). Inner nuclear envelope proteins SUN1 and SUN2 play a prominent role in the DNA damage response. Current Biology, 22(17), 1609–1615.
Liu, C., Yang, Z., Li, D., Liu, Z., Miao, X., Yang, L., Zou, Q., & Yuan, Y. (2015). Overexpression of B2M and loss of ALK7 expression are associated with invasion, metastasis, and poor-prognosis of the pancreatic ductal adenocarcinoma. Cancer Biomarkers, 15(6), 735–743.
Liu, J., Li, H., Sun, L., Feng, X., Wang, Z., Yuan, Y., & Xing, C. (2018). The differential expression of core genes in nucleotide excision repair pathway indicates colorectal carcinogenesis and prognosis. BioMed Research International, 2018, 9651320.
Lundquist, M. R., Goncalves, M. D., Loughran, R. M., Possik, E., Vijayaraghavan, T., Yang, A., Pauli, C., Ravi, A., Verma, A., Yang, Z., Johnson, J. L., Wong, J. C. Y., Ma, Y., Hwang, K. S., Weinkove, D., Divecha, N., Asara, J. M., Elemento, O., Rubin, M. A., … Emerling, B. M. (2018). Phosphatidylinositol-5-phosphate 4-kinases regulate cellular lipid metabolism by facilitating autophagy. Molecular Cell, 70(3), 531-544 e539.
Matsumoto, S., Fischer, E. S., Yasuda, T., Dohmae, N., Iwai, S., Mori, T., Nishi, R., Yoshino, K., Sakai, W., Hanaoka, F., Thoma, N. H., & Sugasawa, K. (2015). Functional regulation of the DNA damage-recognition factor DDB2 by ubiquitination and interaction with xeroderma pigmentosum group C protein. Nucleic Acids Research, 43(3), 1700–1713.
Moller, A., Sirma, H., Hofmann, T. G., Staege, H., Gresko, E., Ludi, K. S., Klimczak, E., Droge, W., Will, H., & Schmitz, M. L. (2003). Sp100 is important for the stimulatory effect of homeodomain-interacting protein kinase-2 on p53-dependent gene expression. Oncogene, 22(54), 8731–8737.
Ni, M., Zhang, W. Z., Qiu, J. R., Liu, F., Li, M., Zhang, Y. J., Liu, Q., & Bai, J. (2014). Association of ERCC1 and ERCC2 polymorphisms with colorectal cancer risk in a Chinese population. Science and Reports, 4, 4112.
Pogue-Geile, K. L., Chen, R., Bronner, M. P., Crnogorac-Jurcevic, T., Moyes, K. W., Dowen, S., Otey, C. A., Crispin, D. A., George, R. D., Whitcomb, D. C., & Brentnall, T. A. (2006). Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Medicine, 3(12), e516.
Raghu, P. (2021). Emerging cell biological functions of phosphatidylinositol 5 phosphate 4 kinase. Current Opinion in Cell Biology, 71, 15–20.
Rameh, L. E., Tolias, K. F., Duckworth, B. C., & Cantley, L. C. (1997). A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature, 390(6656), 192–196.
Richards, M. W., Burgess, S. G., Poon, E., Carstensen, A., Eilers, M., Chesler, L., & Bayliss, R. (2016). Structural basis of N-Myc binding by Aurora-A and its destabilization by kinase inhibitors. Proceedings of the National Academy of Sciences, 113(48), 13726–13731.
Rochman, M., Taher, L., Kurahashi, T., Cherukuri, S., Uversky, V. N., Landsman, D., Ovcharenko, I., & Bustin, M. (2011). Effects of HMGN variants on the cellular transcription profile. Nucleic Acids Research, 39(10), 4076–4087.
Rubinsztein, D. C., Cuervo, A. M., Ravikumar, B., Sarkar, S., Korolchuk, V., Kaushik, S., & Klionsky, D. J. (2009). In search of an “autophagomometer.” Autophagy, 5(5), 585–589.
Said, N. (2016). Roles of SPARC in urothelial carcinogenesis, progression and metastasis. Oncotarget, 7(41), 67574–67585.
Sergeeva, O., & Zatsepin, T. (2021). RNA helicases as shadow modulators of cell cycle. Progression International Journal of Molecular Sciences, 22(6), 2984.
Sharma, S., Mathre, S., Ramya, V., Shinde, D., & Raghu, P. (2019). Phosphatidylinositol 5 phosphate 4-kinase regulates plasma-membrane pip3 turnover and insulin signaling. Cell Reports, 27(7), 1979-1990 e1977.
Sokolova, M., Turunen, M., Mortusewicz, O., Kivioja, T., Herr, P., Vaharautio, A., Bjorklund, M., Taipale, M., Helleday, T., & Taipale, J. (2017). Genome-wide screen of cell-cycle regulators in normal and tumor cells identifies a differential response to nucleosome depletion. Cell Cycle, 16(2), 189–199.
Srivastava, A. K., Yadav, S. S., Mishra, S., Yadav, S. K., Parmar, D., & Yadav, S. (2020). A combined microRNA and proteome profiling to investigate the effect of ZnO nanoparticles on neuronal cells. Nanotoxicology, 14(6), 757–773.
Stijf-Bultsma, Y., Sommer, L., Tauber, M., Baalbaki, M., Giardoglou, P., Jones, D. R., Gelato, K. A., van Pelt, J., Shah, Z., Rahnamoun, H., Toma, C., Anderson, K. E., Hawkins, P., Lauberth, S. M., Haramis, A. P., Hart, D., Fischle, W., & Divecha, N. (2015). The basal transcription complex component TAF3 transduces changes in nuclear phosphoinositides into transcriptional output. Molecular Cell, 58(3), 453–467.
Sumita, K., Lo, Y. H., Takeuchi, K., Senda, M., Kofuji, S., Ikeda, Y., Terakawa, J., Sasaki, M., Yoshino, H., Majd, N., Zheng, Y., Kahoud, E. R., Yokota, T., Emerling, B. M., Asara, J. M., Ishida, T., Locasale, J. W., Daikoku, T., Anastasiou, D., … Sasaki, A. T. (2016). The lipid kinase PI5P4Kbeta Is an intracellular GTP sensor for metabolism and tumorigenesis. Molecular Cell, 61(2), 187–198.
Sun, L. C., & Qian, H. X. (2018). Screening for implicated genes in colorectal cancer using wholegenome gene expression profiling. Molecular Medicine Reports, 17(6), 8260–8268.
Tadesse, S., Yu, M., Kumarasiri, M., Le, B. T., & Wang, S. (2015). Targeting CDK6 in cancer: state of the art and new insights. Cell Cycle, 14(20), 3220–3230.
Wang, D. G., Paddock, M. N., Lundquist, M. R., Sun, J. Y., Mashadova, O., Amadiume, S., Bumpus, T. W., Hodakoski, C., Hopkins, B. D., Fine, M., Hill, A., Yang, T. J., Baskin, J. M., Dow, L. E., & Cantley, L. C. (2019). PIP4Ks suppress insulin signaling through a catalytic-independent mechanism. Cell Reports, 27(7), 1991-2001 e1995.
Wang, H., Nicolay, B. N., Chick, J. M., Gao, X., Geng, Y., Ren, H., Gao, H., Yang, G., Williams, J. A., Suski, J. M., Keibler, M. A., Sicinska, E., Gerdemann, U., Haining, W. N., Roberts, T. M., Polyak, K., Gygi, S. P., Dyson, N. J., & Sicinski, P. (2017). The metabolic function of cyclin D3–CDK6 kinase in cancer cell survival. Nature, 546(7658), 426–430.
Xu, B., Wu, D. P., Xie, R. T., Liu, L. G., & Yan, X. B. (2017). Elevated NDC80 expression is associated with poor prognosis in osteosarcoma patients. European Review for Medical and Pharmacological Sciences, 21(9), 2045–2053.
Yin, J., Chen, G., Liu, Y., Liu, S., Wang, P., Wan, Y., Wang, X., Zhu, J., & Gao, H. (2010). Downregulation of SPARC expression decreases gastric cancer cellular invasion and survival. Journal of Experimental & Clinical Cancer Research, 29, 59.
Zafeer, M., Mahjabeen, I., & Kayani, M. A. (2016). Increased expression of ERCC2 gene in head and neck cancer is associated with aggressive tumors: a systematic review and case-control study. International Journal of Biological Markers, 31(1), e17-25.
Zheng, F., Yue, C., Li, G., He, B., Cheng, W., Wang, X., Yan, M., Long, Z., Qiu, W., Yuan, Z., Xu, J., Liu, B., Shi, Q., Lam, E. W. F., Hung, M.-C., & Liu, Q. (2016). Nuclear AURKA acquires kinase-independent transactivating function to enhance breast cancer stem cell phenotype. Nature Communications, 7(1), 10180.
Acknowledgements
We thank CSIR-IITR confocal microscopy, proteomics, cell culture and RT-PCR facility for their support. Murine embryonic fibroblast (MEF) K-Ras 4B G12V were from Dr Rachel K. Bagni (Frederick National Laboratory for Cancer Research (FNLCR) NCI, USA). CMT-93 were gifted by Dr Florian Greten (Germany) and K-Ras G12D plasmid was from Addgene. We thank Dr Abhilasha Kanaujia for editorial assistance. P.A. and V.K.Y. were supported by CSIR-SRF fellowships. Work in A.K. lab is supported by Wellcome Trust/DBT India Alliance intermediate fellowship (Grant number: IA/16/2/502721) and CSIR intramural funding. A.K. is DBT-Wellcome India Alliance Intermediate fellow. CSIR manuscript number IITR/SEC/2021-2022/57.
Author information
Affiliations
Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, Uttar Pradesh, India
Poorwa Awasthi, Ankur Kumar Srivastava, Vipin Kumar Yadav, Radhika Singh & Amit Kumar
CSIR-Indian Institute of Toxicology Research, Lucknow, Uttar Pradesh, 226001, India
Poorwa Awasthi, Ankur Kumar Srivastava, Vipin Kumar Yadav, Smriti Singh Yadav & Amit Kumar
Institute of Systems Genetics, New York University, New York, NY, USA
Gururaj Rao Kidiyoor
CSIR- Institute of Microbial Technology, Chandigarh, India
Radhika Singh & Amit Kumar
Contributions
PA: planned all experiments, performed all the experiments, analysed the data presented in all figures, assisted in manuscript writing and prepared figures. AKS: data analysis using proteome discoverer, figure presentation, Run HRMS equipment. SSY: data analysis using proteome discoverer. VKY: assisted in HRMS experiment. RS: assisted in autophagy experiment. GRK: data analysis. AK: conceptualized study, provided resource, designed, planned experiments, wrote the manuscript.
Corresponding author
Correspondence to Amit Kumar.
Ethics declarations
Conflict of interest
The authors declare non-financial interests that are directly or indirectly related to the work submitted for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (XLSX 106 KB)
Supplementary file2 (XLSX 13 KB)
Supplementary file3 (XLSX 15 KB)
Supplementary file4 (XLSX 144 KB)
Rights and permissions
About this article
Cite this article
Awasthi, P., Srivastava, A.K., Yadav, V.K. et al. Proteome profiling of phosphatidylinositol-5-phosphate 4-kinase type 2A and 2B knockdown cells identify modifications in key regulators involved in cell homeostasis and genome integrity. GENOME INSTAB. DIS. (2022). https://doi.org/10.1007/s42764-022-00060-7
Received28 November 2021
Revised15 January 2022
Accepted25 January 2022
Published22 February 2022
DOIhttps://doi.org/10.1007/s42764-022-00060-7
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkKeywords
Phosphoinositide kinase
Cancer
DNA repair
Genome instability
Transcription
Cell migration
Metastasis
Post-translational modifications








用户登录
还没有账号?
立即注册