Epithelial tumor compartment- and adjacent stromal compartment-specific expression of PARP1 in different anatomical sites in patients with HGSOC
Original Research Paper
Genome Instability & Disease 3, 201–208 (2022)
Abstract
High-grade serous ovarian carcinoma (HGSOC) is responsible for the majority of ovarian cancer-associated deaths. PARP (poly-(ADP-ribose) polymerase) inhibitors (PARPi) (olaparib, rucaparib and niraparib) have been approved in recent years to be used in both the frontline setting as maintenance therapy and in the recurrent setting for patients with ovarian cancer (OC). Previous studies have reported an increased expression of PARP1, a sensor for DNA damage, in OC; however, its compartment (epithelial tumor vs stroma)-, anatomical site (fallopian tube, omentum, ovary and serous tubal in situ carcinoma)- and histotype-specific expression have not been studied. Here, we showed that PARP1 protein levels are higher in epithelial tumors compared to adjacent non-malignant stroma at four different anatomical locations in HGSOC, with the most significant difference in PARP1 levels in omental metastases. Furthermore, we found that PARP1 protein levels are elevated in tumors relative to normal tissue in the fallopian tube, omentum and ovary in patients with HGSOC, again with the most significant difference in the omentum. We also showed that PARP1 expression is increased in a malignant and invasive stage in mouse ovarian surface epithelium cells, and that PARP1 mRNA levels are lower in mucinous histotype compared to serous histotype of epithelial OC. These findings indicate that a better understanding of compartment-, anatomical location- and histotype-specific changes in PARP1 levels in OC is needed since PARP inhibitors are currently being used in the treatment of patients with OC.
Introduction
High-grade serous ovarian carcinoma (HGSOC) is the most frequent and lethal type of ovarian carcinoma, and it is responsible for the majority of ovarian cancer-associated deaths in women (Siegel et al., 2018; Torre et al., 2018). HGSOC has a high proportion of stromal cells besides epithelial tumor cells in the tumor microenvironment (Eckert et al., 2019). These stromal cells support tumor cells adjacent to them and co-evolve with the epithelial compartment during cancer progression and metastasis (Eckert et al., 2019; Kalluri, 2016; Polyak et al., 2009). However, a better understanding of molecular differences between epithelial tumor cells and adjacent stromal cells in the tumor microenvironment, especially in terms of drug vulnerabilities, is needed to develop more effective targeted therapies.
Standard frontline care for patients with ovarian cancer remains surgical debulking and platinum-based chemotherapy (such as with cisplatin) (Berkel et al., 2020); thus, overall survival has not changed significantly for several decades, despite a better understanding of the molecular mechanisms in HGSOC. More than 80% of women with HGSOC treated with these options ultimately experience disease recurrence, limiting the efficacy of the current treatment strategies (Salani et al., 2011; Zivanovic et al., 2009). PARP (poly-(ADP-ribose) polymerase) inhibitors (PARPi) have been approved in recent years to be used in both the frontline setting as maintenance therapy and in the recurrent setting for patients with ovarian cancer (Kristeleit et al., 2017; Moore et al., 2018; Pujade-Lauraine et al., 2017). Cytotoxicity induced by PARPi is considered to be caused, at least in part, by the trapping of PARP1 on DNA lesions, limiting its activity in DNA damage response (Kristeleit et al., 2017; Moore et al., 2018; Pujade-Lauraine et al., 2017).
HGSOC is almost always TP53-mutant, and approximately 50% of patients have deficiencies in homologous recombination (HR) DNA repair of DNA double-strand breaks (DSB), most frequently due to the presence of mutations in BRCA1/2 (Konstantinopoulos et al., 2015). Tumors with deficiencies in HR display favourable responses to cisplatin chemotherapy and PARPi (Konstantinopoulos et al., 2015; Lord & Ashworth, 2017; Scott et al., 2015). PARP enzymes are involved in DNA repair by promoting base excision repair and alternative end-joining pathways and by limiting non-homologous end-joining (NHEJ) pathway (Scott et al., 2015). The sensitivity of HR-deficient cells to PARPi relies on overactivation of the NHEJ pathway, impaired DNA replication fork protection and persistence of unrepaired collapsed forks (Ceccaldi et al., 2015; Sanij et al., 2020; Scott et al., 2015; Swisher et al., 2017).
Since PARP inhibition with drugs such as olaparib is currently in use in the treatment of patients with HGSOC (Berkel et al., 2020), PARP1 levels in epithelial tumor cells and adjacent stromal cells are of high clinical importance. Here, we showed that PARP1 protein levels are higher in epithelial tumor cells compared to adjacent non-malignant stromal cells at 4 different anatomical locations in HGSOC, with the highest difference in PARP1 levels observed in omental metastases. Similarly, we found that PARP1 protein levels are higher in tumors relative to normal tissues in the fallopian tube, omentum and ovary in patients with HGSOC. Furthermore, we showed that PARP1 expression is increased in the malignant and invasive stage compared to pre-neoplastic, non-malignant stage or to the neoplastic, pre-invasive stage in mouse ovarian surface epithelium cells. Finally, we found that PARP1 mRNA levels are lower in mucinous histotype (which are known to have a low response rate to chemotherapy) compared to serous histotype of epithelial ovarian cancer. Data presented in the current study highlight that a complete understanding of compartment-, anatomical location- and histotype-specific changes in PARP1 levels in OC is needed.
Materials and methods
Proteome data
Proteomics data for high-grade serous ovarian carcinoma (HGSOC) patients, acquired using liquid chromatography (LC)-mass spectrometry (MS) from formalin-fixed, paraffin-embedded (FFPE) specimens were accessed from MaxQB—The MaxQuant DataBase (Max-Planck-Institut für Biochemie) (Eckert et al., 2019) (n = 107). [Dataset can be accessed through http://maxqb.biochem.mpg.de/mxdb/project/show/9373012627500]. This dataset contains protein expression levels in log10 scale for epithelial tumor cells and surrounding non-malignant stromal cells at four different anatomical locations in patients with HGSOC (namely, omental metastasis, serous tubal in situ carcinoma (STIC), invasive fallopian tube (FT) lesions and invasive ovarian lesions) (Eckert et al., 2019).
Transcriptome data
Following transcriptomics datasets from Gene Expression Omnibus (GEO) were used in this study: GSE24789 (Creekmore et al., 2011, n = 36), GSE14764 (Denkert et al., 2009, n = 80), GSE20565 (Meyniel et al., 2010, n = 172), GSE2109 (n = 57), GSE26193 (Mieulet et al., 2021, n = 107), GSE30161 (Ferriss et al., 2012, n = 58), GSE44104 (Wu et al., 2015, n = 60), GSE6008 (Wu et al., 2016, n = 103), GSE6822 (n = 74), GSE8842 (Marchini et al., 2008, n = 83) and GSE9891 (Tothill et al., 2008, n = 285). These datasets were loaded into R using the curated Ovarian Data Bioconductor package (Ganzfried et al., 2013). Each sample was indicated as a data point in the plots to show relative sample sizes (n). More experimental details of these datasets can be accessed in GEO using given accession numbers.
Data analysis and visualization
Following R packages used in the data analysis and visualization (R Core Team, 2020): tidyverse (Wickham et al., 2019), readxl (Wickham & Bryan, 2019), ggpubr (Kassambara, 2020), tidytext (Silge & Robinson, 2016) and rmarkdown (Allaire et al., 2021).
Following convention for star symbols indicating statistical significance (p values) was used in the analysis of data in the present study: non-significant (ns): p > 0.05; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001 (ggpubr package) (Kassambara, 2020). The normality test for the data used in each plot was performed using ggqqplot() (Quantile–Quantile plot) and shapiro.test() (Shapiro–Wilk normality test) functions in R (from ggpubr and stats R packages, respectively) (Kassambara, 2020; R Core Team, 2020). When data is normally distributed, we used Student’s t test to compare group means; otherwise, we performed the analysis using two-sample Wilcoxon test (Mann–Whitney test). No observations were excluded from the analyses.
Results
PARP1 protein levels are higher in tumors compared to adjacent stroma at 4 different anatomical locations in HGSOC, with the most significant difference in PARP1 levels in omental metastases
To investigate if PARP1 protein levels are different between epithelial tumor cells and adjacent non-malignant stromal cells present in the surrounding microenvironment, in patients with high-grade serous ovarian carcinoma (HGSOC), we analyzed the proteomics data by Eckert et al. (2019) obtained using LC–MS. We found that PARP1 protein levels are higher in tumor cells compared to adjacent non-malignant stromal cells, at all four different anatomical locations (fallopian tube, omentum, ovary and STIC (serous tubal in situ carcinoma)) in patients with HGSOC (Fig. 1A). Between these anatomical sites, the omentum represents a metastatic site called omental metastases, the other three (fallopian tube, ovary and STIC) represent primary tumor tissues. In terms of anatomical locations, the highest difference in PARP1 protein levels between epithelial tumor compartment and non-malignant stromal compartment was observed for omentum (p = 9.3e−08), followed by ovary (p = 3.2e−05), STIC (p = 0.0025) and fallopian tube (p = 0.002) (Fig. 1A). In other words, the difference in PARP1 protein levels between the epithelial tumor compartment and non-malignant stromal compartment is the most significant in omental metastases compared to that observed in other primary tumor tissues.
Fig. 1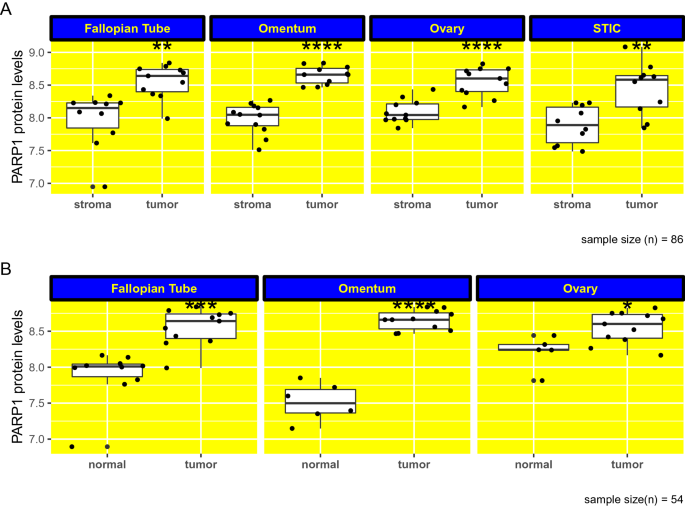
PARP1 protein levels are higher in tumors compared to adjacent non-malignant stroma in HGSOC. A PARP1 protein levels are higher in tumors compared to adjacent non-malignant stroma at 4 different anatomical locations (fallopian tube, omentum, ovary and STIC) in high-grade serous ovarian carcinoma (HGSOC), with the most significant difference in PARP1 levels in omental metastases. Protein levels in y axis are shown in log10 scale. B PARP1 protein levels are higher in tumors compared to normal tissue in fallopian tube, omentum and ovary in patients with HGSOC. Protein levels in y axis are shown in log10 scale. non-significant [ns]: p > 0.05; ∗: p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. STIC serous tubal in situ carcinoma. (n = 107)
Full size imagePARP1 protein levels are higher in tumors compared to normal tissue in the fallopian tube, omentum and ovary in patients with HGSOC
Next, we analyzed PARP1 expression at the protein level between HGSOC tumors and corresponding healthy tissues in 3 different anatomic sites, namely, fallopian tube, omentum and ovary. We found that, for each of these sites, PARP1 protein levels are increased in tumor samples compared to samples from healthy tissues (Fig. 1B). The most significant difference in PARP1 protein levels between tumor and normal samples based on p values was observed for omentum (p = 3.3e−05), followed by fallopian tube (p = 0.00021) and ovary (p = 0.028) (Fig. 1B). Similar to the previous observation that differences in PARP1 protein levels between epithelial tumor compartment and non-malignant stromal compartment are the most significant for omental metastases (Fig. 1A), PARP1 protein level differences between normal and tumor tissues is the most significant also in the omentum. Collectively, these data may point that the contribution of PARP1, at least in part, to omental metastases.
PARP1 expression is increased in malignant and invasive stage in mouse ovarian surface epithelium cells at the mRNA level
Since we observed the most significant increases in PARP1 protein levels in omental metastases (Fig. 1), we wanted to see if its expression at the mRNA level also increases depending on different levels of malignancy. To analyze PARP1 expression levels at different stages of malignancy, we used transcriptome data from mouse ovarian surface epithelial (MOSE) cells (Creekmore et al., 2011). Here, we used data from mice, since similar data from patients is not currently available. These mouse cells undergo spontaneous transformation in cell culture with increasing passage numbers, and represent phenotypes from pre-neoplastic, non-malignant to malignant and invasive (Creekmore et al., 2011). Since we observed the most significant difference in PARP1 levels in omental metastases when comparing its levels between tumors and adjacent stroma, we wanted to see if PARP1 expression increases in parallel to increasing malignancy. We found that PARP1 mRNA levels are indeed higher in malignant and invasive stage (late cells, passage 136, 142, and 143) compared to both pre-neoplastic, non-malignant stage (early cells, passage 13, 14, and 15) (p = 0.003) and neoplastic, pre-invasive stage (intermediate cells, passage 63, 71, and 73) (p = 0.0036) (Fig. 2).
Fig. 2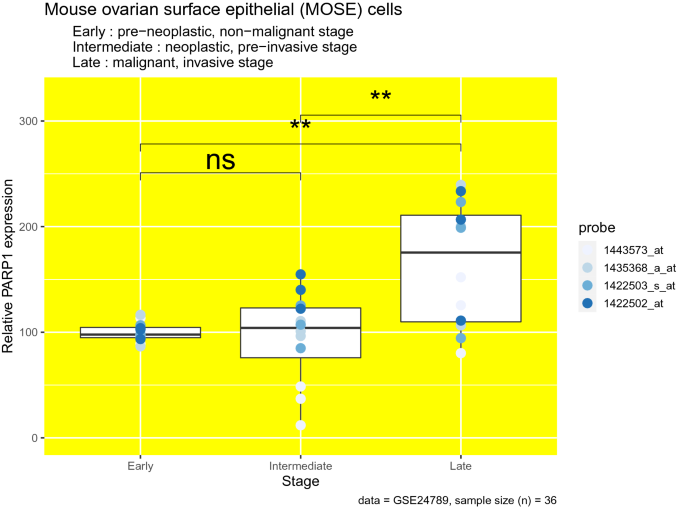
PARP1 expression is increased in malignant and invasive stage in mouse ovarian surface epithelium (MOSE) cells. Relative PARP1 expression levels (PARP1 mRNA molecule count) at different transformation stages (different stages of malignancy) in MOSE cells. Different colors given in the figure legend indicate different probes used to measure PARP1 expression. (n = 36). non-significant [ns]: p > 0.05; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001
Full size imagePARP1 mRNA levels are lower in mucinous histotype compared to serous histotype of epithelial ovarian cancer
Next, we analyzed if PARP1 mRNA levels differ between different histological types (histotypes) of epithelial ovarian cancer (it is classified into different histotypes: clear cell, endometrium, mucinous, serous and also very rare malignant Brenner tumors) (Berkel & Cacan, 2021). We found that PARP1 expression is lower in mucinous histotype compared to serous histotype in 5 out of 6 datasets in which data is available for these histotypes (p values: 0.048, 0.0024, 0.027, 0.0017 and 9.1e−05) (Fig. 3). Between other histological types, there seems to be no significant change in PARP1 mRNA levels, which is consistent between datasets (Fig. 3).
Fig. 3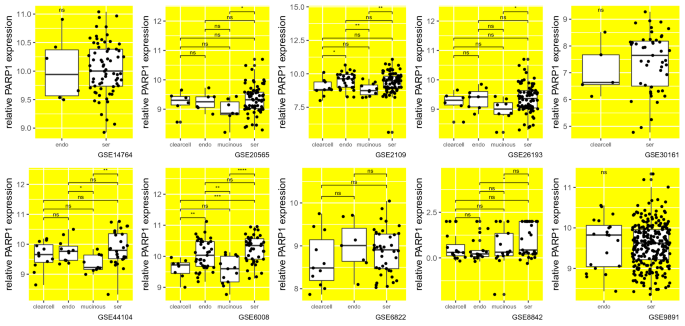
PARP1 mRNA levels are lower in mucinous histotype compared to serous histotype of epithelial ovarian cancer. Relative PARP1 expression levels (PARP1 mRNA molecule count) of different histological types (histotypes) of epithelial ovarian cancer (EOC); clearcell: clear cell; endo: endometrium, mucinous; ser: serous. Each sample was indicated as a data point in the plots to show relative sample sizes. Each subplot shows data from a different dataset whose accession ID was given at the bottom. non-significant [ns]: p > 0.05; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001
Full size imageDiscussion
In this study, we showed that PARP1 expression is increased in the epithelial tumor compartment compared to adjacent non-malignant stromal compartment at 4 different anatomical sites (fallopian tube, omentum, ovary and STIC (serous tubal in situ carcinoma)) in patients with HGSOC. We observed the most significant difference in PARP1 protein levels between the two compartments in omental metastases compared to that in primary tumor tissues (fallopian tube, ovary and STIC). These data show that epithelial tumor cells have higher PARP1 protein expression compared to adjacent stromal cells in the tumor microenvironment, independently of their anatomical location. This is of high clinical importance since PARP inhibitors including olaparib are currently being used in the treatment of patients with ovarian cancer in both the frontline setting as maintenance therapy and in the recurrent setting (Kristeleit et al., 2017; Moore et al., 2018; Pujade-Lauraine et al., 2017). Because the difference in PARP1 protein levels between tumor and stroma is the most significant in omental metastases, PARP inhibition may promote synthetic lethality more favorably in metastases compared to primary tumor sites. This is because the epithelial tumor compartment will be potentially more preferentially targeted than the stromal compartment in omental metastases by the inhibition of PARP. It can also be speculated that particularly increased PARP1 expression in epithelial tumor cells compared to adjacent cells may contribute to the heterogeneity in terms of drug sensitivity in the cells present in the tumor microenvironment. Specific targeting of PARP1 in tumor cells instead of targeting this protein in the whole tumor may be more effective in limiting tumor progression with less side effects. However, further research is needed to make stronger inferences. Single-cell studies performed with samples from the ovarian tumor microenvironment may provide more data on the cell type-dependent expression of PARP1 and ultimately help us to identify specific cells to target with PARPi, improving the clinical efficacy of these drugs (Berkel & Cacan, 2019).
Similarly, we observed that PARP1 protein levels are higher in tumors compared to normal tissue in the fallopian tube, omentum and ovary in patients with HGSOC, again with the highest difference observed in omentum/omental metastases. Therefore, we can state that the most significant increase in PARP1 protein levels between the two compartments in the course of cancer progression occurs in omental metastasis compared to primary tumor sites, pointing to the potential contribution of PARP1, at least in part, to advanced disease and metastasis.
We also found that PARP1 expression is increased in malignant and invasive mouse ovarian surface epithelial cells compared to those with pre-neoplastic, non-malignant phenotype or with neoplastic, pre-invasive phenotype in vitro. These data are in line with a recent study showing that PARP1 overexpression promotes viability, migration, invasion, and tube formation in ovarian cancer cells (Chang et al., 2021). Wei et al. showed that hepatocyte growth factor (HGF) administration increases PARP1 levels and enhances cell invasion in a concentration- and time-dependent manner in ovarian cancer cells in vitro (Wei et al., 2018). They also reported that the silencing of PARP1 significantly reduces the impact of HGF on invasiveness in ovarian cancer cells, pointing to the role of PARP1 in the development of an invasive phenotype (Wei et al., 2018). Same group also found that PARP1 may enhance angiogenesis in epithelial ovarian cancer by upregulating VEGF-A (Wei et al., 2016). Therefore, differential expression of PARP1 in terms of the compartment, anatomical site and the degree of malignancy may be of high clinical importance, and PARP inhibition in specific cells may be more effective in limiting tumor progression and thus improving survival rates in patients with ovarian cancer. Therefore, the preliminary data presented in the current study may provide the basis for the further exploration of the role of PARP1 in HGSOC progression and for the development of more targeted and personalized treatment strategies.
Our analysis also pointed out that mostly lower PARP1 mRNA levels are observed in the mucinous histotype of epithelial ovarian cancer compared to the serous histotype. This difference in PARP1 levels may contribute, at least to a certain extent, to the differences between these two histological types in terms of survival and response to chemotherapy and PARP inhibition (Overall, patients with mucinous histotype have a better prognosis compared to those with serous histotype in the early stage; however, patients with mucinous type have a worse prognosis at advanced stage compare to patients with serous histotype) (Monk et al., 2019; Peres et al., 2019; Simons et al., 2017). In other words, varying clinical success rates in the treatment of different histological types of epithelial ovarian cancer with PARP inhibitors may be attributed, at least in part, to the differential expression of PARP1 in these histotypes. A complete picture of PARP1-mediated cellular events in different histotypes may help us to optimize treatment plans for patients with different subtypes of OC and to develop more tailored treatment strategies.
Combined, we showed that PARP1 protein levels are higher in tumor samples compared to both adjacent non-malignant stroma or normal ovaries, in patients with HGSOC, with the most significant difference in omental metastases, compared to that in primary tumor tissues. This points to a potential mechanism of PARP1 in promoting malignancy in ovarian cancer; however, mechanistic studies are needed to test these hypotheses. Supporting, we showed that malignant mouse ovarian surface epithelial cells have higher PARP1 expression compared to those with low malignancy. Furthermore, we observed differential levels of PARP1 in various histological types of epithelial ovarian cancer, possibly contributing to varying degrees of malignancy observed in these histotypes. This preliminary study highlights the need for a complete understanding of compartment-, anatomical location- and histotype-specific changes in PARP1 levels in ovarian cancer. For instance, how PARP1 expression is upregulated in ovarian tumors compared to normal ovaries or surrounding stromal cells, or the mechanisms by which PARP1 promotes malignancy in ovarian cancer should be studied in more detail. This is of high clinical importance since PARP inhibitors are currently being used in the treatment of ovarian cancer, and mechanisms leading to PARPi resistance still remain to be identified. Since we analyzed publicly available data in the present study, more research both in vitro and in vivo is needed to confirm and support the findings of this study and to provide mechanistic details of PARP1-mediated events in tumor progression in ovarian cancer.
Data availability statement
No new data was generated during this study. R code written to analyze datasets was given as a supplementary file.
References
Allaire, J. J., Xie, Y., McPherson, J., et al. (2021). rmarkdown: Dynamic documents for R. R Package Version, 2, 10.
Berkel, C., & Cacan, E. (2019). Single-cell epigenomics in cancer research. Biomedical Journal of Scientific and Technical Research, 21, 15966–15973.
Berkel, C., & Cacan, E. (2021). GAB2 and GAB3 are expressed in a tumor stage-, grade- and histotype-dependent manner and are associated with shorter progression-free survival in ovarian cancer. Journal of Cell Communication and Signaling, 15(1), 57–70. https://doi.org/10.1007/s12079-020-00582-3
Berkel, C., Kucuk, B., Usta, M., et al. (2020). The effect of olaparib and bortezomib combination treatment on ovarian cancer cell lines. European Journal of Biology, 79(2), 115–123.
Ceccaldi, R., Liu, J. C., Amunugama, R., et al. (2015). Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature, 518(7538), 258–262. https://doi.org/10.1038/nature14184
Chang, H., Zhang, X., Li, B., et al. (2021). PARP1 is targeted by miR-519a-3p and promotes the migration, invasion, and tube formation of ovarian cancer cells. Cancer Biotherapy and Radiopharmaceuticals. https://doi.org/10.1089/cbr.2020.4394.Advanceonlinepublication
Creekmore, A. L., Silkworth, W. T., Cimini, D., et al. (2011). Changes in gene expression and cellular architecture in an ovarian cancer progression model. PLoS ONE, 6(3), e17676. https://doi.org/10.1371/journal.pone.0017676
Denkert, C., Budczies, J., Darb-Esfahani, S., et al. (2009). A prognostic gene expression index in ovarian cancer—Validation across different independent data sets. The Journal of Pathology, 218(2), 273–280. https://doi.org/10.1002/path.2547
Eckert, M. A., Coscia, F., Chryplewicz, A., et al. (2019). Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature, 569(7758), 723–728. https://doi.org/10.1038/s41586-019-1173-8
Ferriss, J. S., Kim, Y., Duska, L., et al. (2012). Multi-gene expression predictors of single drug responses to adjuvant chemotherapy in ovarian carcinoma: Predicting platinum resistance. PLoS ONE, 7(2), e30550. https://doi.org/10.1371/journal.pone.0030550
Ganzfried, B. F., Riester, M., Haibe-Kains, B., et al. (2013). curatedOvarianData: Clinically annotated data for the ovarian cancer transcriptome. Database: the Journal of Biological Databases and Curation, 2013, bat013. https://doi.org/10.1093/database/bat013
Kalluri, R. (2016). The biology and function of fibroblasts in cancer. Nature Reviews. Cancer, 16(9), 582–598. https://doi.org/10.1038/nrc.2016.73
Kassambara A (2020) ggpubr: 'ggplot2' based publication ready plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr.
Konstantinopoulos, P. A., Ceccaldi, R., Shapiro, G. I., et al. (2015). Homologous recombination deficiency: Exploiting the fundamental vulnerability of ovarian cancer. Cancer Discovery, 5(11), 1137–1154. https://doi.org/10.1158/2159-8290.CD-15-0714
Kristeleit, R., Shapiro, G. I., Burris, H. A., et al. (2017). A phase I–II study of the oral parp inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 23(15), 4095–4106. https://doi.org/10.1158/1078-0432.CCR-16-2796
Lord, C. J., & Ashworth, A. (2017). PARP inhibitors: Synthetic lethality in the clinic. Science (new York, NY), 355(6330), 1152–1158. https://doi.org/10.1126/science.aam7344
Marchini, S., Mariani, P., Chiorino, G., et al. (2008). Analysis of gene expression in early-stage ovarian cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 14(23), 7850–7860. https://doi.org/10.1158/1078-0432.CCR-08-0523
Meyniel, J. P., Cottu, P. H., Decraene, C., et al. (2010). A genomic and transcriptomic approach for a differential diagnosis between primary and secondary ovarian carcinomas in patients with a previous history of breast cancer. BMC Cancer, 10, 222. https://doi.org/10.1186/1471-2407-10-222
Mieulet, V., Garnier, C., Kieffer, Y., et al. (2021). Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer. Scientific Reports, 11(1), 4219. https://doi.org/10.1038/s41598-021-83685-0
Monk, B. J., Randall, L. M., & Grisham, R. N. (2019). The evolving landscape of chemotherapy in newly diagnosed advanced epithelial ovarian cancer. American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology Annual Meeting, 39, e141–e151. https://doi.org/10.1200/EDBK_239007
Moore, K., Colombo, N., Scambia, G., et al. (2018). Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. The New England Journal of Medicine, 379(26), 2495–2505. https://doi.org/10.1056/NEJMoa1810858
Peres, L. C., Cushing-Haugen, K. L., Köbel, M., et al. (2019). Invasive epithelial ovarian cancer survival by histotype and disease stage. Journal of the National Cancer Institute, 111(1), 60–68. https://doi.org/10.1093/jnci/djy071
Polyak, K., Haviv, I., & Campbell, I. G. (2009). Co-evolution of tumor cells and their microenvironment. Trends in Genetics: TIG, 25(1), 30–38. https://doi.org/10.1016/j.tig.2008.10.012
Pujade-Lauraine, E., Ledermann, J. A., Selle, F., et al. (2017). Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet. Oncology, 18(9), 1274–1284. https://doi.org/10.1016/S1470-2045(17)30469-2
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Salani, R., Backes, F. J., Fung, M. F., et al. (2011). Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. American Journal of Obstetrics and Gynecology, 204(6), 466–478. https://doi.org/10.1016/j.ajog.2011.03.008
Sanij, E., Hannan, K. M., Xuan, J., et al. (2020). CX-5461 activates the DNA damage response and demonstrates therapeutic efficacy in high-grade serous ovarian cancer. Nature Communications, 11(1), 2641. https://doi.org/10.1038/s41467-020-16393-4
Scott, C. L., Swisher, E. M., & Kaufmann, S. H. (2015). Poly (ADP-ribose) polymerase inhibitors: Recent advances and future development. Journal of Clinical Oncology, 33(12), 1397–1406. https://doi.org/10.1200/JCO.2014.58.8848
Siegel, R. L., & Miller, K. D. (2018). Jemal A (2018) Cancer statistics. CA: A Cancer Journal for Clinicians, 68(1), 7–30. https://doi.org/10.3322/caac.21442
Silge J, Robinson D (2016) tidytext: Text mining and analysis using tidy data principles in R. _JOSS_, 1(3). https://doi.org/10.21105/joss.00037.
Simons, M., Massuger, L., Bruls, J., et al. (2017). Relatively poor survival of mucinous ovarian carcinoma in advanced stage: A systematic review and meta-analysis. International Journal of Gynecological Cancer, 27(4), 651–658. https://doi.org/10.1097/IGC.0000000000000932
Swisher, E. M., Lin, K. K., Oza, A. M., et al. (2017). Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. The Lancet. Oncology, 18(1), 75–87. https://doi.org/10.1016/S1470-2045(16)30559-9
Torre, L. A., Trabert, B., DeSantis, C. E., et al. (2018). Ovarian cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68(4), 284–296. https://doi.org/10.3322/caac.21456
Tothill, R. W., Tinker, A. V., George, J., et al. (2008). Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clinical Cancer Research, 14(16), 5198–5208. https://doi.org/10.1158/1078-0432.CCR-08-0196
Wei, W., Li, Y., Lv, S., et al. (2016). PARP-1 may be involved in angiogenesis in epithelial ovarian cancer. Oncology Letters, 12(6), 4561–4567. https://doi.org/10.3892/ol.2016.5226
Wei, W., Lv, S., Zhang, C., et al. (2018). Potential role of HGF-PARP-1 signaling in invasion of ovarian cancer cells. International Journal of Clinical and Experimental Pathology, 11(7), 3310–3317.
Wickham H, Bryan J (2019) readxl: Read excel files. R package version 1.3.1. https://CRAN.R-project.org/package=readxl.
Wickham, H., et al. (2019). Welcome to the tidyverse. Journal of Open Source Software, 4(43), 1686. https://doi.org/10.21105/joss.01686
Wu, R., Zhai, Y., Kuick, R., et al. (2016). Impact of oviductal versus ovarian epithelial cell of origin on ovarian endometrioid carcinoma phenotype in the mouse. The Journal of Pathology, 240(3), 341–351. https://doi.org/10.1002/path.4783
Wu, Y. H., Chang, T. H., Huang, Y. F., et al. (2015). COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPβ pathway and PDK1 stabilization. Oncotarget, 6(27), 23748–23763. https://doi.org/10.18632/oncotarget.4250
Zivanovic, O., Aldini, A., Carlson, J. W., et al. (2009). Advanced cytoreductive surgery: American perspective. Gynecologic Oncology, 114(2 Suppl), S3–S9. https://doi.org/10.1016/j.ygyno.2008.11.033
Acknowledgements
CB is funded by the 2211-E programme of The Scientific and Technological Research Council of Turkey.
Author information
Authors and Affiliations
Department of Molecular Biology and Genetics, Tokat Gaziosmanpasa University, Tokat, 60250, Turkey
Caglar Berkel & Ercan Cacan
Corresponding authors
Correspondence to Caglar Berkel or Ercan Cacan.
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berkel, C., Cacan, E. Epithelial tumor compartment- and adjacent stromal compartment-specific expression of PARP1 in different anatomical sites in patients with HGSOC. GENOME INSTAB. DIS. 3, 201–208 (2022). https://doi.org/10.1007/s42764-022-00075-0
Received26 May 2022
Revised13 July 2022
Accepted15 July 2022
Published27 July 2022
Issue DateAugust 2022
DOIhttps://doi.org/10.1007/s42764-022-00075-0
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkKeywords
DNA repair
Olaparib
Omentum
Ovarian cancer
Ovary
PARP1








用户登录
还没有账号?
立即注册