FAM135B: a new player in DNA damage response
Review Article
Genome Instability & Disease 3, 238–240 (2022)
Abstract
The ATM (Ataxia telangiectasia mutated) kinase and its complex with TIP60 could rapidly respond to DNA double-strand breaks. But the molecular mechanism remains elusive. New research identifies FAM135B as a novel regulator of TIP60-ATM axis. FAM135B interacts with and maintains the TIP60-ATM reservoir in resting condition, whereas it dissociates from the complex and degrades, allowing cells to respond to DNA damage. This work reveals a direct link between the steady-state organization of ATM and the kinetics of its activation after DNA damage.
DNA damage during cell growth, such as spontaneous damage, ionizing radiation, chemical and other genetic toxicants, poses a great threat to genetic stability and cell survival (Gospodinov & Herceg, 2013). When cell senses DNA damage, especially double-strand breaks (DSBs), the DNA damage response (DDR) is stimulated to activate the downstream repair pathways. This process involves a variety of cellular events, such as phosphorylation, ubiquitination, acetylation and so forth (Jackson & Bartek, 2009). Among the numerous DDR factors, ATM is one of the apical kinases in response to DSBs (Menolfi & Zha, 2019). Upon DSBs induction, the MRN (Mre11/Rad50/Nbs1) complex recognizes the breakage sites and recruits ATM, leading to the trans-autophosphorylation of ATM at serine 1981 (Awasthi et al., 2015; Shiloh & Ziv, 2013). Additional ATM autophosphorylation events occur at serine 367, serine 1893 and serine 2996 (Awasthi et al., 2015; Kozlov et al., 2011; Sun et al., 2007). Autophosphorylation of the ATM dimer occurs after its association with the MRN complex and before the formation of a fully active monomer (Shiloh & Ziv, 2013). Acetylation of ATM by TIP60 is also required for ATM activation and DSBs repair (Sun et al., 2005; Xu et al., 2012). TIP60 and ATM form a complex in which TIP60 associates with the highly conserved FATC domain of ATM at the C-terminus (Jiang et al., 2006; Sun et al., 2005). This interaction promotes acetylation of lysine 3016 on ATM, and mutation of this site renders ATM unresponsive to DSBs (Sun et al., 2007). Currently, how the TIP60-ATM complex is regulated remains unclear.
A new study from the Qimin Zhan group at Peking University (Zhang et al., 2022), published recently in Clinical and Translational Medicine, identifies FAM135B (family with sequence similarity 135 member B, also known as C8ORFK32) as a novel regulator of TIP60-ATM axis. This work reveals a direct link between the steady-state organization of ATM and the kinetics of its activation after DNA damage. Previous work from this group demonstrated that FAM135B is a novel cancer-related gene, harboring 6.8% mutations in oesophageal squamous-cell carcinoma (ESCC) samples and is associated with worse survival of patients with ESCC (Song et al., 2014). They recently find FAM135B promotes cell proliferation through direct interaction with growth factor GRN, forming a feedforward loop with AKT/mTOR signaling (Dong et al., 2021). Independently, another group shows that FAM135B regulates PI3K/Akt/mTOR signaling pathway, and silencing of FAM135B enhances radio-sensitivity of ESCC (Bi et al., 2021). These findings lay solid groundwork to study the role of FAM135B in DDR and genome instability.
Consistent with previous work (Dong et al., 2021; Song et al., 2014), the authors show that FAM135B suppression sensitizes cancer cells to treatment of chemotherapy drugs, whereas overexpression of FAM135B causes drug resistance. They speculate that FAM135B promotes DSBs repair. Further, they confirm FAM135B can orchestrate the ATM signaling to deal with genotoxic stress. This conclusion comes from the observation that the decrease of γH2AX is delayed with FAM135B suppression, while γH2AX is removed more rapidly with FAM135B overexpression. To examine the link between ATM and FAM135B, the authors search multiple databases of protein–protein interactions for FAM135B. Surprisingly, they find that TIP60 is a potential interactor of FAM135B. Indeed, the authors demonstrate that FAM135B directly interacts with the chromo-domain (CHD) of TIP60 and promotes its acetylase activity. This leads to the increase of ATM phosphorylation. More importantly, the function of FAM135B in DDR relies on TIP60 (Zhang et al., 2022).
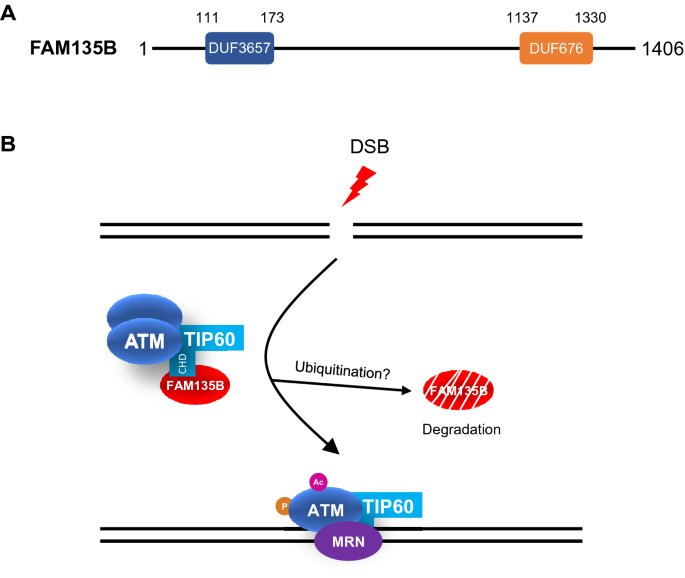
Despite its importance in cancer and high conservation in vertebrates, FAM135B remains largely uncharacterized. The FAM135B gene, located in chromosome 8, encodes a protein with 1406 amino acids, and it is predicted to contain a hydrolase-like DUF3657 domain at the N-terminus and a lipase-like DUF676 domain close to the C-terminus (Fig. 1A). Although the authors show clearly that FAM135B interacts with the CHD domain of TIP60 in vitro and in cells (Zhang et al., 2022), they have not identified the interaction region on FAM135B. The role of the DUF3657 domain and the DUF676 domain in FAM135B-TIP60 interaction and in DDR requires further investigation.
Structure of FAM135B and its function in DNA damage response. A Predicted structure of FAM135B. It has a DUF3657 domain at the N-terminus and a DUF676 domain close to the C-terminus (data from http://pfam.xfam.org/). B Model of FAM135B in DNA damage response. FAM135B interacts with the chromo-domain (CHD) of TIP60 and promotes the assembly of TIP60-ATM complex. Upon damage, FAM135B is dissociated from TIP60-ATM complex and degraded, allowing the acetylation (Ac) and phosphorylation (P) activation of ATM and downstream signaling
Moreover, overexpression of FAM135B promotes TIP60-ATM interaction, while suppression of FAM135B attenuates this interaction. Intuitively, one would expect FAM135B associates with TIP60-ATM complex during DDR. Surprisingly, the authors find that the protein level of FAM135B is reduced during DNA damage and recovered after repair. They propose that DSBs induces the dissociation and degradation of FAM135B from TIP60-ATM, releasing the CHD domain of TIP60 and potentially the acetylated ATM to translocate to DSBs (Zhang et al., 2022). This ‘licensing’ model may explain why the TIP60-ATM could rapidly respond to DNA damage and the counter-intuitive behavior of FAM135B during DDR (Fig. 1B).
Over the years, genetic screens and cell biology experiments have identified many regulators for TIP60 such as H3K9me3, RNF8, PRMT, ATF2 and ATF3 (Bhoumik et al., 2008; Cui et al., 2015; Goel et al., 2020; Goodarzi et al., 2008; Ikura et al., 2007). These factors regulate TIP60 either at the transcriptional level or through post-translational modifications. Unlike other regulators of TIP60, the FAM135B complex with TIP60-ATM is pre-assembled independent of DSBs. Furthermore, FAM135B directly binds to the CHD domain of TIP60 and inhibits the activation of ATM by TIP60. Therefore, the authors identify FAM135B as a new player in DDR through TIP60, adding an additional layer of regulation in DSBs repair. The most important question is how FAM135B is dissociated from TIP60-ATM and degraded. Proteins can be degraded through ubiquitin—proteasome, lysosome or autophagy. The ubiquitin-dependent signaling during the DDR is initiated by two E3 ubiquitin ligases RNF8 and RNF168 which are critical for signal transduction and activation of repair pathways (Nowsheen et al., 2019). It is possible that RNF8 and RNF168 or other uncharacterized E3 ubiquitin ligase(s) regulate FAM135B dynamics during DDR. This may address how the DSBs signal is transmitted to FAM135B. In addition, FAM135B shows high expression in ESCC (Song et al., 2014), and has been reported to regulate PI3K/Akt/mTOR pathway (Bi et al., 2021). Whether the function of TIP60-ATM regulated by FAM135B is involved in the PI3K/Akt/mTOR pathway is currently unanswered. Meanwhile, FAM135B is predicted to play a role in cellular lipid metabolism, based on its structure. It is of interest to investigate the cellular and physiological functions of FAM135B, and connect it with its role in DDR.
In summary, the authors thoroughly characterize a novel FAM135B-TIP60-ATM complex that is pre-assembled and ‘licensed’ for DDR. The key component FAM135B seems to be an important therapeutic target for ESCC, with implication for other cancer types. Therefore, developing small-molecule inhibitor and inhibitory antibody against FAM135B is of great significance for anti-tumor therapy, at least for ESCC.
References
Awasthi, P., Foiani, M., & Kumar, A. (2015). ATM and ATR signaling at a glance. Journal of Cell Science, 128, 4255–4262.
Bhoumik, A., Singha, N., O’Connell, M. J., & Ronai, Z. A. (2008). Regulation of TIP60 by ATF2 modulates ATM activation. Journal of Biological Chemistry, 283, 17605–17614.
Bi, L., Wang, H., & Tian, Y. (2021). Silencing FAM135B enhances radiosensitivity of esophageal carcinoma cell. Gene, 772, 145358.
Cui, H., Guo, M., Xu, D., Ding, Z. C., Zhou, G., Ding, H. F., Zhang, J., Tang, Y., & Yan, C. (2015). The stress-responsive gene ATF3 regulates the histone acetyltransferase Tip60. Nature Communications, 6, 6752.
Dong, D., Zhang, W., Xiao, W., Wu, Q., Cao, Y., Gao, X., Huang, L., Wang, Y., Chen, J., Wang, W., et al. (2021). A GRN autocrine-dependent FAM135B/AKT/mTOR feedforward loop promotes esophageal squamous cell carcinoma progression. Cancer Research, 81, 910–922.
Goel, P. N., Grover, P., & Greene, M. I. (2020). PRMT5 and Tip60 modify FOXP3 function in tumor immunity. Critical Reviews in Immunology, 40, 283–295.
Goodarzi, A. A., Noon, A. T., Deckbar, D., Ziv, Y., Shiloh, Y., Löbrich, M., & Jeggo, P. A. (2008). ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Molecular Cell, 31, 167–177.
Gospodinov, A., & Herceg, Z. (2013). Chromatin structure in double strand break repair. DNA Repair, 12, 800–810.
Ikura, T., Tashiro, S., Kakino, A., Shima, H., Jacob, N., Amunugama, R., Yoder, K., Izumi, S., Kuraoka, I., Tanaka, K., et al. (2007). DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Molecular and Cellular Biology, 27, 7028–7040.
Jackson, S. P., & Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature, 461, 1071–1078.
Jiang, X., Sun, Y., Chen, S., Roy, K., & Price, B. D. (2006). The FATC domains of PIKK proteins are functionally equivalent and participate in the Tip60-dependent activation of DNA-PKcs and ATM. Journal of Biological Chemistry, 281, 15741–15746.
Kozlov, S. V., Graham, M. E., Jakob, B., Tobias, F., Kijas, A. W., Tanuji, M., Chen, P., Robinson, P. J., Taucher-Scholz, G., Suzuki, K., et al. (2011). Autophosphorylation and ATM activation: Additional sites add to the complexity. Journal of Biological Chemistry, 286, 9107–9119.
Menolfi, D., & Zha, S. (2019). ATM, DNA-PKcs and ATR: Shaping development through the regulation of the DNA damage responses. Genome Instability & Disease, 1, 47–68.
Nowsheen, S., Deng, M., & Lou, Z. (2019). Ubiquitin and the DNA double-strand break repair pathway. Genome Instability & Disease, 1, 69–80.
Shiloh, Y., & Ziv, Y. (2013). The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nature Reviews Molecular Cell Biology, 14, 197–210.
Song, Y., Li, L., Ou, Y., Gao, Z., Li, E., Li, X., Zhang, W., Wang, J., Xu, L., Zhou, Y., et al. (2014). Identification of genomic alterations in oesophageal squamous cell cancer. Nature, 509, 91–95.
Sun, Y., Jiang, X., Chen, S., Fernandes, N., & Price, B. D. (2005). A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proceedings of the National Academy of Sciences, 102, 13182–13187.
Sun, Y., Xu, Y., Roy, K., & Price, B. D. (2007). DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Molecular and Cellular Biology, 27, 8502–8509.
Xu, Y., Xu, C., & Price, B. D. (2012). Mechanistic links between ATM and histone methylation codes during DNA repair. Progress in Molecular Biology and Translational Science, 110, 263–288.
Zhang, K., Wu, Q., Liu, W. Z., Wang, Y., Zhao, L. M., Chen, J., Liu, H. Y., Liu, S. Q., Li, J. T., Zhang, W. M., et al. (2022). FAM135B sustains the reservoir of Tip60-ATM assembly to promote DNA damage response. Clinical and Translational Medicine. https://doi.org/10.1002/ctm2.945
Funding
The study was funded by Basic and Applied Basic Research Foundation of Guangdong Province (Grant no. 2020A1515110542) and Shenzhen Bay Laboratory Open Fund (Grant no. SZBL2020090501004) to LD.
Author information
Authors and Affiliations
Shenzhen Bay Laboratory, Shenzhen, 518132, China
Chunyu Song & Lin Deng
Department of Biochemistry and Molecular Biology, Capital Medical University, Beijing, 100069, China
Chunyu Song & Lin Deng
Corresponding author
Correspondence to Lin Deng.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, C., Deng, L. FAM135B: a new player in DNA damage response. GENOME INSTAB. DIS. 3, 238–240 (2022). https://doi.org/10.1007/s42764-022-00079-w
Received16 July 2022
Revised04 August 2022
Accepted15 August 2022
Published22 August 2022
Issue DateOctober 2022
DOIhttps://doi.org/10.1007/s42764-022-00079-w
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkKeywords
FAM135B
DNA double-strand breaks
TIP60
ATM
DNA replication








用户登录
还没有账号?
立即注册