DNA damage repair and cancer immunotherapy
Review Article
Genome Instability & Disease (2023)
Abstract
Cells are exposed to multiple endogenous and exogenous stresses daily, some of which can induce DNA damage, a major source of genomic instability. To deal with damaged DNA and maintain genomic integrity, cells have evolved complicated DNA damage repair (DDR) machinery. Impaired DDR pathways can lead to the accumulation of DNA damage and genomic instability, a risk factor for carcinogenesis. If cancer occurs, DDR is normally associated with increased survival of tumor cells and the development of treatment resistance. Increasingly, evidence shows that the activity of DDR pathways not only impact the outcomes of cytotoxic treatments, but also multiple aspects of anti-tumor immunity. As such, DDR deficiency is emerging as a promising prognostic factor in predicting the therapeutic outcomes of immunotherapy. Accordingly, modulation of DDR pathways can improve immunotherapeutic efficiency in cancer treatment. In this review, we outline the mechanisms of DNA damage, the DDR pathways which counteract them and summarize the association between altered DDR pathways and cancer. Additionally, we highlight DDR deficiency in the context of cancer immunity, and its potential applications in the combined treatment of cancer.
DNA damage and DNA damage repair
DNA damage is an unavoidable cellular event. Tens of thousands of cells experience daily DNA damage in the human body, which can be caused by a variety of endogenous factors (Tubbs & Nussenzweig, 2017), such as DNA replication errors, intracellular oxidative stress, and metabolic products, or exogenous factors (Friedberg, 2008; Waterman et al., 2020) mainly consisting of ultraviolet radiation (UV), ionizing radiation (IR), and genotoxic chemicals (Fig. 1). Endogenous DNA damage can lead to transcriptional inhibition or replication disorders (Brueckner et al., 2007; Gregersen & Svejstrup, 2018; Tufegdžić Vidaković et al., 2020; Xu et al., 2017). Whereas exogenous sources can inflict single- or double-strand breaks (SSB or DSB), with DSBs being the most deleterious form of DNA damage (Waterman et al., 2020). Complex and coordinated mechanisms have evolved to deal with DNA damage and maintain genomic integrity, which is called DNA damage repair (DDR) (Jackson & Bartek, 2009; Ribezzo et al., 2016). If not repaired timely and accurately, these can result in genomic instability, an enabling factor for carcinogenesis (Lord & Ashworth, 2012; Roos et al., 2016). Cells with modest DNA damage may survive through DNA repair, while cells with severe DNA damage may be urged to undergo programmed cell death, such as apoptosis (Gasser & Raulet, 2006) (Fig. 1).
Fig. 1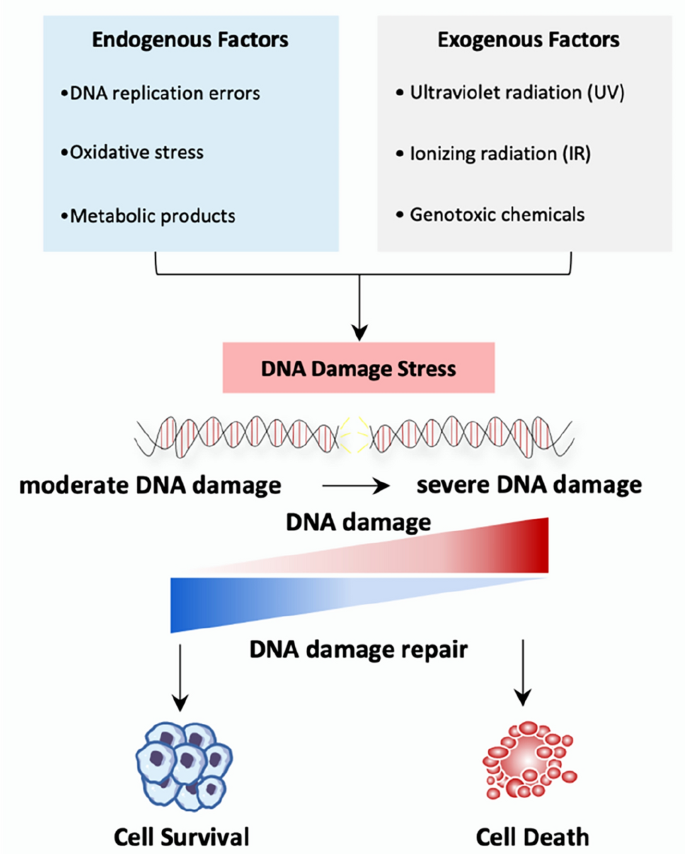
Source of DNA damage and the outcomes. DNA damage can be caused by a variety of endogenous factors such as DNA replication errors, intracellular oxidative stress and metabolic products, or exogenous factor mainly consisting of UV, IR, and genotoxic chemicals. Endogenous factors generally induce moderate DNA damage, while exogenous factors lead to severe DNA damage. Cells with moderate DNA damage may survive through DNA repair, while cells with severe DNA damage may undergo cell death
Diverse DDR pathways are activated in response to different forms of DNA damage, including: mismatch repair (MMR), nucleotide excision repair (NER), base excision repair (BER), homologous recombination repair (HRR) and non-homologous end-joining repair (NHEJ). These mechanisms can repair simple DNA damage independently, or repair more complex lesions in a coordinated manner. The details of these different DDR pathways are described in further detail below.
Mismatch repair (MMR)
MMR is primarily responsible for removing mismatched bases and small loops formed from small insertions and deletions, which arise mainly due to replication errors (Kunkel & Erie, 2015) (Fig. 2). This mechanism is essential to ensure the fidelity of DNA replication and prevent mutations in the genome. When replication errors occur, MutSα (MSH2-MSH6) or MutSβ (MSH2-MSH3) and MutLα (MLH1-PMS2) move to the mismatch site and form a sliding clamp loader (Bradford et al., 2020; Yang et al., 2022), which subsequently recruits ATP/ADP cofactors to modulate the protein–protein or protein-DNA interactions (Hura et al., 2013; Mendillo et al., 2010). Proliferating cellular nuclear antigen (PCNA) is then loaded onto the DNA, through replication factor C (RFC) and then interacts with MutLα and MutS (MutSα or MutSβ) (Kang et al., 2019). Subsequently, PCNA activates MutLα (Pluciennik et al., 2010), leading to MutLα-mediated cutting of the DNA strand to create a gap, followed by exonuclease 1 (EXO1) mediated excision and the formation of a replication protein A (RPA)-coated single-strand gap (Genschel & Modrich, 2009). Finally, DNA polymerase δ (Polδ), in cooperation with RFC and PCNA, promotes DNA resynthesis to restore the integrity of the duple (Constantin et al., 2005), while DNA ligase I rejoins the single-strand ends together (Reyes et al., 2021).
Fig. 2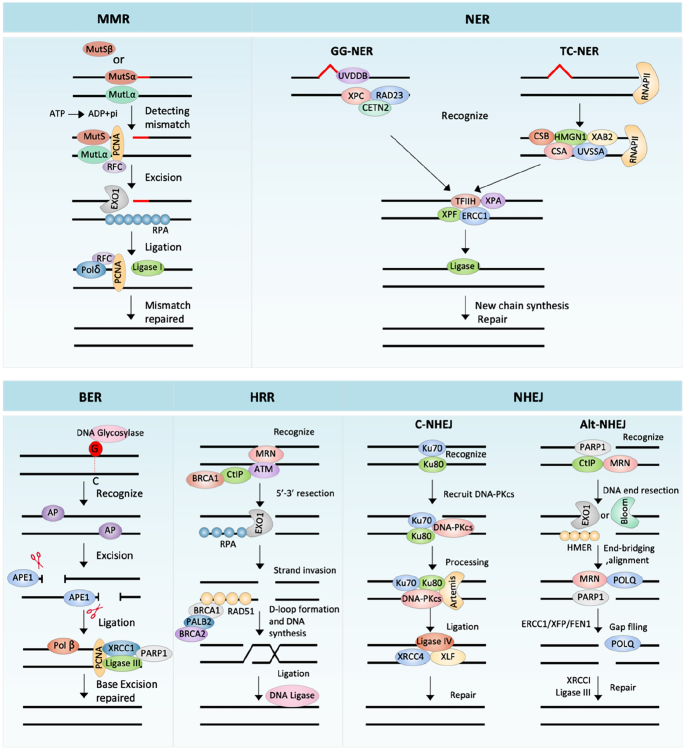
The process and major components of MMR, NER, BER, HRR and NHEJ. See text for detailed descriptions
MMR deficiency (dMMR) can lead to microsatellite instability (MSI) and increased tumor mutational burden (TMB) (Lizardo et al., 2020; Yen et al., 2021). A strong clinical relevance between colorectal cancer and MMR status has long been noted. dMMR is detected in up to 15% of colorectal cancers, of which 12% are caused by sporadic inactivation and 3% are associated with Lynch syndrome (Boland & Goel, 2010). It is now known that dMMR also exists in many other cancer types, such as: endometrial (Zighelboim et al., 2007), gastric (Cortes-Ciriano et al., 2017), small intestine (Le et al., 2017), cervical (Bonneville et al., 2017; Cortes-Ciriano et al., 2017), prostate (Burger et al., 2006; Cortes-Ciriano et al., 2017), ovarian (Cortes-Ciriano et al., 2017; Murphy & Wentzensen, 2011), cholangiocarcinoma (Bonneville et al., 2017) and uterine sarcoma (Kang et al., 2019; Le et al., 2017). Sporadic dMMR cancers and Lynch syndrome can be caused by epigenetic silencing of the gene encoding DNA mismatch repair protein MLH1 (MLH1), and germline defects in one of the four MMR genes MLH1, MSH2, MSH6 or PMS2 (Boland & Goel, 2010). Given its correlation with cancer, MSI/MMR detection has been recommended for inclusion in routine screening for colorectal cancers by the guidelines of the National Cancer Comprehensive Network (NCCN) (Benson et al., 2021).
Nucleotide excision pathways (NER)
NER can repair a large amount of cumulative damage, in the region of 25 to 30 nucleotide fragments (Torres-Ramos et al., 2000), caused by exposure to environmental factors or anticancer drugs (Fig. 2). NER constitutes two sub-pathways: transcription-coupled (TC)-NER and global genome (GG)-NER. TC-NER is initiated by the stagnation of RNA polymerase II molecules (RNAPII) (Nakazawa et al., 2020), followed by the recruitment of DNA damage repair proteins CSA, CSB and accessory proteins (UVSSA, XAB2 and HMGN1) to the lesion site (Tiwari et al., 2021). GG-NER is triggered by two protein complexes; XPC, RAD23A or RAD23B and CETN2 containing complex; and UV-DDB complex containing DDB1, CUL4A or CUL4B, RBX1 and DDB2 (Matsumoto et al., 2019; Sugasawa et al., 2005). After that, the transcription factor II H (TFIIH) complex and DNA damage recognition and repair factor (XPA) unwind the DNA helix around the damage, followed by the removal of the damaged nucleotide mediated by the ERCC1-XPF enzyme complex (Geng et al., 2020). Finally, the polymerase δ/ε (Pol δ/ε) fills the gap which are subsequently joined by DNA ligase I (Fousteri & Mullenders, 2008).
NER deficiency has been linked to two important human diseases: xeroderma pigmentosum (XP) and Cockayne syndrome (CS) (Vélez-Cruz et al., 2013). The association between NER gene polymorphism and specific cancers has been extensively studied, for example approximately 4.5% of all human tumors contain ERCC1 mutations (Jager et al., 2019; Ni et al., 2014).
Base excision repair (BER)
BER repairs damage to DNA bases modified by a series of chemicals causing deamination, alkylation or oxidation (Bauer et al., 2015) (Fig. 2). BER starts with recognizing modified bases by DNA glycosylase, which produces apurinic/apyrimidinic (AP) sites (Mullins et al., 2019). AP endonuclease 1 (APE1) can effectively cleave the generated AP sites to form DNA gaps (Abbotts & Madhusudan, 2010). DNA Polymerase β (Polβ) fills the gap with the correct base, which is followed by gap connection, mediated by DNA ligase III and DNA repair proteins XRCC1, PARP-1 and PCNA (Beard et al., 2019; Demin et al., 2021). Abnormal expression or genetic defects of important BER pathway genes, such as: APE1 (Lin et al., 2020), OGG1 (Leitner-Dagan et al., 2012), XRCC1 (Sak et al., 2005) and PARP-1 (Berndt et al., 2007; Riffell et al., 2012), are related to cancer occurrence and a poor prognosis for patients. Therefore, BER is considered as an important target for cancer treatments.
Homologous recombination repair (HRR)
DSBs can be repaired by HRR (Fig. 2), which uses the homologous DNA sequence from the sister chromatid as a template, thus occurring mainly in the S and G2 phases of the cell cycle (Daley et al., 2014), although G1 phase repair has been also reported (Yilmaz et al., 2021). During HRR, the MRE11–RAD50–NBS1 (MRN) complex senses DSBs and promotes the excision of the 5´ end with the help of BRCA1 and CtIP, which leads to the generation of 3´-terminal single-stranded DNA (ssDNA) (Batenburg et al., 2019). Replication protein A (RPA) then binds to the ssDNA, removing the secondary structures of ssDNA (Spegg et al., 2023), and subsequently being replaced by RAD51 with the help of the mediating protein BRCA1, BRCA2 and PALB2 (Bhat & Cortez, 2018). RAD51 nucleoprotein filament invades the homologous DNA strand and forms a D-loop (Wright & Heyer, 2014), which captures the second end and produces an intermediate, dissolved by DNA helicase or nuclease to produce a no-cross or cross product (Wright et al., 2018). For synthesis-dependent strand annealing, the new chain is shifted from the D-loop and annealed to the end of other side of the DSB (Paliwal et al., 2014). The D-loop forms a replication fork, to ensure synthesis of the following chain and leading chain, before DNA ligase-mediated DNA connection (Wright et al., 2018).
PI3K protein kinase family member, ATM (Ataxia-Telangiectasia Mutated), is a key regulator of multiple signaling cascades in HRR pathways. It is activated at the early stage of DSB, following the recruitment of the MRN complex at sites of DNA damage (Lee & Paull, 2004). Mechanically, MRE11 and RAD50 can bind the end of DSBs, and NBS1 connects with ATM (Lee & Paull, 2004). Upon ATM activation, H2AX is phosphorylated (Lee & Paull, 2004). ATM has a broad spectrum of downstream targets, including proteins involved in DNA repair, cell cycle inspection, apoptosis and other related pathways, such as p53 (Nakamura, 1998), MDM2 (Chibaya et al., 2021), CHK2 (Wang et al., 2020b), BRCA1 (Foo et al., 2021), and NBS1 (Kijas et al., 2015). Additionally, ATM can regulate short range DNA terminal excision through the MRN complex and CtIP, as well as conducting remote terminal excision through Bloom syndrome protein (BLM) and EXO1, leading to the promoted strand invasion mediated by RAD51 (Weitering et al., 2021).
The serine/threonine-protein kinase ATR (ataxia-telangiectasia mutated- and Rad3-related) is another key regulator of the HRR pathway. RPA can recruit ATR to DNA lesions, consequently activating ATR (Haahr et al., 2016). Once activated, the downstream target protein CHK1 is subsequently activated, thereby protecting cells from replication stress (da Costa et al., 2023). ATR can transmit signals of DNA damage during S and G2/M checkpoints and DNA repair pathways. Activated ATR can also regulate the HRR pathway through direct regulation of BRCA1 (Foo et al., 2021). BRCA1/BRCA2 protein mutations are the main biomarkers of HRR defects, initially identified in breast and ovarian cancers (Miki et al., 1994; Wooster et al., 1995), and are subsequently found also in pancreatic and prostate cancers (Nguyen et al., 2020).
Non-homologous end joining (NHEJ)
Unlike HRR, NHEJ does not require a repair template (Fig. 2). NHEJ can be divided into two distinct sub-pathways: Classical NHEJ (C-NHEJ) and alternative NHEJ (Alt-NHEJ).
Despite being occur throughout the cell cycle. C-NHEJ acts mostly in G1 (Liang et al., 2021). During C-NHEJ, heterodimers of Ku proteins are recruited, forming a basket-shaped structure with a central cavity containing the dsDNA ends (Downs & Jackson, 2004). C-NHEJ is initiated when Ku70 and Ku80 dimers bind to DSB ends with a high affinity and specificity. In this process, Ku complexes protect the DNA from further resection, and recruit additional NHEJ factors, including the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), nuclease Artemis, DNA ligase IV and the associated scaffolding factors XRCC4, XRCC4-like factor (XLF) and paralogue of XRCC4 and XLF (PAXX) (Abbasi et al., 2021).
DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) belongs to the PI3K protein kinase family, which senses DNA damage by interacting with the C-terminal domain of Ku80 (Gell & Jackson, 1999). After binding to the Ku complex, DNA-PKcs is autophosphorylated and activated, thereby activating Artemis (Jiang et al., 2015), a member of the β-CASP family of nucleases. The latter protein can remove 5´ overhangs in the presence of DNA-PKcs (Ma et al., 2002). When the ends of DNA breaks are removed by Artemis, the repair step of C-NHEJ is performed by DNA polymerase (Lieber, 2023). Finally, the ligase IV-XRCC4-XLF complex repairs the break (Jayaram et al., 2008). Cancer predisposition of Bloom's syndrome and myeloid leukemias exhibit increased C-NHEJ activity (Brady et al., 2003).
Alt-NHEJ is a backup repair pathway (Boulton & Jackson, 1996), which can be detected when C-NHEJ or HR pathway is compromised. Compared with C-NHEJ, Alt-NHEJ is more prone to errors. In the first step of Alt-NHEJ, MRN and CtIP are recruited to DSB by PARP1, leading to DNA end resection (Xie et al., 2009). The newly generated ssDNA 3' overhang is elongated by EXO1 or BLM/DNA2, which is then coated by proteins, such as HMCES (Shukla et al., 2020). The following end-bridging and alignment step is mediated by the coordinated action of PARP1, MRN and POLQ, another protein recruited to the DSB by PARP1, leading to the creation of non-homologous 3' tails (Yousefzadeh et al., 2014). These tails need to be removed by ERCC1/XPF, and FEN1, before the POLQ-mediated gap filling of Alt-NHEJ (Patterson-Fortin & D'Andrea, 2020). Finally, the end is connected by LIG3/XRCC1 (Patterson-Fortin & D'Andrea, 2020). Growing evidence shows that up-regulation of this error-prone pathway is related to the acquisition of new genetic changes that lead to the onset and progression of cancer. For example, Tobin et al. demonstrated that, compared with non-neoplastic breast epithelial cell MCF10A, estrogen receptor and progesterone receptor positive MCF7 breast cancer cells contain the increased activity of Alt-NHEJ (Tobin et al., 2012). Alt-NHEJ repair activity was also found to be increased in chronic myeloid leukemia (CML) BCR-ABL positive cells (Sallmyr et al., 2008).
DDR is essential for resolving DNA damage to maintain genomic stability. Next, we will discuss the relationship between DNA damage repair and cancer development, focusing on cancer immunity.
DNA damage repair and cancer
Genomic instability is a hallmark of most cancer cells, mainly due to increased DNA damage or defects in DNA repair pathways (Huang & Zhou, 2021), and the accumulation of genomic instability promotes tumor growth. DDR gene mutations have been detected in many types of tumors and mounting evidence suggests that DDR defects are the major factors conferring chemoresistance and radio-resistance in cancer cells (Wang et al., 2012; Furgason & Bahassi, 2013). Such knowledge has been extensively reviewed previously. It should be noted that genomic instability has been shown to alter the tumor immune microenvironment and activate anti-tumor immunity in recent studies. In addition, DDR deficiencies are closely related to the outcomes of patients receiving immunotherapy. Therefore, we will focus on the association between tumor DDR and the immune response.
DNA damage repair and anti-tumor immunity
Cytotoxic T cells are an essential effector of anti-tumor immunity. In this regard, to produce effective anti-tumor activities, dendritic cells (DC) need to take up tumor antigen, subsequently cross-presented for CD8 + T cell priming (Jhunjhunwala et al., 2021). Additionally, tumor cells can directly present antigens to T cells, also leading to their activation. Tumor antigenicity, the ability of tumor cells to present human leukocyte antigen (HLA)-restricted epitopes of immunogenicity (Chabanon et al., 2021), is thus a major determinate of the vulnerability of T cell-mediated tumor destruction. Tumors with strong antigenicity can be killed by an effective immune response, while tumor cells with weak antigenicity can escape the surveillance of the immune system (Kroemer et al., 2022). In addition to the antigenicity of tumor cells, immune checkpoints regulate T cell activity during an immune response, which also plays an essential role in tumor immunity (He & Xu, 2020). As such, immune checkpoint inhibitors (ICIs), for example anti-PD-L1/PD-1 and anti-CTLA-4 drugs, have attracted significant attention because such treatments significantly improve survival of metastatic solid tumor patients (He & Xu, 2020). Furthermore, the roles of tumor cell death signal or tumor aneuploid in regulating immune system response has attracted increasing attention (Davoli et al., 2017; Kroemer et al., 2013; Upadhyay et al., 2021). In addition to members of the adaptive immunity, the cGAS-STING signaling pathway, an important part of innate immunity, has also attracted widespread attention for their positive roles in anti-tumor immune response (Ablasser & Chen, 2019).
Recently, increasing evidence shows that targeting DDR pathways in tumor cells can encourage anti-tumor immunity (Pilger et al., 2021). Moreover, the combination of DDR targeted therapy and immunotherapy has also emerged in clinical research and has obtained promising therapeutic effects (Pilger et al., 2021). Below, we will review the mechanism of DDR deficiency-induced anti-tumor immune responses, including: regulating the antigenicity of tumor cells, the expression level of immune checkpoints, the tumor cell death signals, the degree of tumor aneuploid and the activation of the cGAS-STING signaling pathway.
Antigenicity
Studies have shown that tumor genome instability is associated with enhanced tumor antigenicity (Mardis, 2019) (Fig. 3a). Given that DDR deficiency promotes tumor genome instability, dysregulated DDR may elevate antigenicity. Indeed, MSI-induced by dMMR can generate new epitopes, form tumor neoantigens and make tumors immunogenic, leading to high tumor mutation burden (TMB) (Baretti & Le, 2018; Marabelle et al., 2020). Due to enhanced immunogenicity of tumors by dMMR, it is not surprising to see great therapeutic benefit in dMMR patients who received immunotherapies. Consequently, MSI/dMMR are now used as biomarkers for anti-PD-1/PD-L1 immunotherapy (Marabelle et al., 2020). It has been shown previously that anti-PD-1/PD-L1 treatment, although promising in the treatment of solid tumors, has little effect on colorectal cancers (Feng et al., 2021). Intriguingly, anti-PD-1/PD-L1 have a higher response rate (partial response rate = 30%) in dMMR colorectal patients (Overman et al., 2017). Similar results were observed in MSI/dMMR patients with pancreatic cancer (Grant et al., 2021), glioma (Das et al., 2022) and triple-negative breast cancer (Yen et al., 2021). Mutations of key genes in the MMR pathway, such as MLH1, MSH2, MSH6, PMS2 or EOX1, result in dMMR, which lead to a high mutation load, the formation of new epitopes, and the production of new antigens (Rospo et al., 2019; Turajlic et al., 2017).
Fig. 3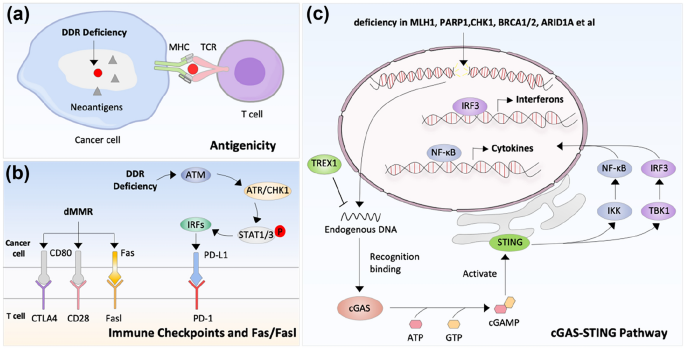
DNA damage repair and anti-tumor immunity. a. DDR deficiency can generate new epitopes, form tumor neoantigens and make tumors immunogenic. The circle means neoantigens that can be recognized by T cells, while and triangles represents other neoantigens that can’t be recognized by T cells. b. DDR deficiency can regulate expression of immune checkpoints, or Fas/FasL system-mediated death. c. DDR deficiency activate cGAS-STING pathway. The functional processes of cGAS-STING include: (1) cGAS recognizes cytoplasmic DNA and generates cyclic GMP-AMP (cGAMP); (2) cGAMP binds to STING and changes STING structure, and activate TANK-binding kinase 1 (TBK1) and inhibitor of nuclear factor kappa-B kinase (IKK); (3) activate NF-kB and IRF3
POLE and POLD1 encode the catalytic subunits of Polε and Polδ, and thus play important roles in NER. It has been shown recently that tumors with POLE and POLD1 mutations tended to acquire SNV or short insertions/deletions (Rospo et al., 2019). These genomic changes can lead to novel putative neoantigens which potentially trigger the immune system (Ma et al., 2022; Rospo et al., 2019). It should be also noted that the levels of additional key components in NER, such as ERCC1, XPA, and XPC, were found to be negatively associated with neoantigen expression (Li et al., 2021). These data suggested that NER pathway deficiency is related to tumor antigenicity.
The loss of another POL family member POLQ in human and mouse cells leads to the increased sensitivity to ionizing radiation and DNA double-strand breaking agents (Yousefzadeh et al., 2014), which mainly attributes to the decreased ability of POLQ in regulating Alt-NHEJ pathway. Interestingly, Huang et al. recently show that patients with defective POLQ (E/Q mutation) have favorable survival, which is correlated with higher TMB scores (Huang et al., 2020). Therefore, Alt-NHEJ deficiency may also contribute to tumor antigenicity.
Recent studies have also shown that tumors with mutations in the chromatin remodeling complex (SWI/SNF) genes exhibit a high mutation burden and genomic instability (Botta et al., 2021). For example, relatively high mutation rates were detected in the chromatin remodeling complex subunit ARID1A in colorectal cancer (Mehrvarz Sarshekeh et al., 2021). ARID1A interacts with MSH2 for mismatch repair during DNA replication (Shen et al., 2018). Patients with ARID1A gene mutations exhibit high TMB, a high frequency of frame shift mutations and increased neoantigens generation (Mehrvarz Sarshekeh et al., 2021).
Furthermore, MUTYH encodes DNA glycosylase in the BER pathway (Nakamura et al., 2021b). Tumors with MUTYH gene mutations show genomic instability and a hypermutation phenotype (Rospo et al., 2019), which is accompanied with the infiltration of NK, CD3 + and CD8 + T cells (Jiang et al., 2021). These data suggest that BER pathway defects also influence anti-tumor immunity.
The association between HRR deficiency and tumor antigenicity has been intensively studied. For example, both BRCA1 and BRCA2 are key genes in the HRR pathway. BRCA1/BRCA2 mutations or hypermethylation of the BRCA1 promoter are positively correlated with neoantigen production in breast cancer (Connor et al., 2017; McAlpine et al., 2012; Nolan et al., 2017; Pettitt et al., 2020).
Defects in individual DDR pathways may lead to a higher dependence on the remaining intact DDR pathways, while multiple defects in different pathways can lead to increased genomic instability. In agreement with this, compared with wild type or single repair pathway mutation patients, the TMB and neoantigen load of patients with HRR-MMR or HRR-BER deficiencies are higher (Wang et al., 2018).
DDR deficiency-caused TMB and neoantigens may drive effective anti-tumor immunity, leading to sustained clinical response. As such, TMB and neoantigen have been used as reasonable predictors for the immunotherapy. However, of the produced neoantigens, only a miniscule fraction can be recognized by T cells. Therefore, TMB and neoantigens are biomarkers only with moderate clinical value when used alone. It still remains challenging to identify immune-related somatic mutations.
Immune checkpoints
PD-L1 is a well-known immune checkpoint molecule in inhibiting T cell activation and can be expressed on tumors. Previous studies have shown that DNA damage and DDR deficiency can regulate the expression of PD-L1 (Fig. 3b) (Permata et al., 2019; Sato et al., 2017; Sun et al., 2021). The upregulation of PD-L1 depends on the activation of the ATM/ATR/C HK1 axis (Sato et al., 2017). Mechanically, DNA damage-induced ATM/ATR/CHK1 activation further activates STAT1/3 and increases the expression of IRF1, leading to the upregulation of PD-L1 levels. BRCA1 or Ku70/Ku80 deficiency can also induce the upregulation of CHK1-dependent PD-L1 expression under DNA damage conditions (Sato et al., 2017). In addition, the DDR repair pathway can regulate PD-L1 through the cGAS-STING pathway. For example, PARP or CHK1 inhibitors promote the activation of the STING-TBK1-IRF3 pathway, which elevate the expression of PD-L1 and IFNβ (Sen et al., 2019). Whereas ATM deficiency can also increase the expression of type I IFN and PD-L1 expression in an alternative pathway, depending on the activation of TBK1 and SRC (Zhang et al., 2019).
In addition to regulating antigenicity, chromatin modifying protein ARID1A has been reported to regulate the expression of PD-L1. Loss of function in ARID1A results in inefficient DDR, leading to genomic instability and a T cell inflammatory microenvironment with relatively high PD-L1 expression (Tokunaga et al., 2020).
Other DNA repair pathways have also been shown to regulate the expression of immune checkpoints. For instance, in response to H2O2-induced oxidative stress, DNA glycosidase NTH1 or OGG1 in the BER pathway can be activated, which subsequently up-regulates PD-L1 expression in an ATR-CHK1 kinase activity-dependent manner (Permata et al., 2019). In addition, dMMR featured tumors exhibit relatively high MSI and TMB, and usually have a continuous response to ICIs, with high T cell infiltration and PD-L1 expression (Liu et al., 2019; Parvathareddy et al., 2021; Sloan et al., 2017).
DDR deficiency can also regulate additional immune checkpoint genes, such as CD80 (B7-1). CD80 is a co-stimulatory molecule known for its role in T-cell activation. In sporadic colorectal cancer, expression of CD80 is higher in patients with dMMR than those patients without dMMR (Scarpa et al., 2015). These data suggest that dMMR patients may be sensitive to anti-CD80 treatments.
As indicated above, the expression levels of immune checkpoints can be regulated by DNA damage or DDR deficiency. Nonetheless, the expression of immune checkpoints exhibit marked spatial and temporal heterogeneity. For example, PD-L1 expression showed significant spatial heterogeneity between baseline primary and metastatic tumors (61% concordance), and temporal heterogeneity between tumors before and after chemotherapy (57–63% concordance) (Zhou et al., 2020). Therefore, although PD-L1 protein expression emerged as the first potential predictive biomarker for sensitivity to immune checkpoint blockade, further research requests to identify the optimal location and time for the detection of these biomarkers.
Cell death signals
Severe DNA damage may cause cell death. It has been shown that DDR deficiency leads to multiple types of DNA damages, among which DSB is closely related to cell death. The dying cells can produce the Damage-related molecular patterns (DAMPs) molecules, which can be recognized by Toll-like receptors (TLRs), RIG-1-like receptors (RLRs) or NOD-like receptors (NLRs), thus stimulating immune activity (Roh & Sohn, 2018). For example, in the late stage of cellular demise, mobility group frame 1 (HMGB1), a non-histone chromatin related protein, is released which also acts as a DAMPs molecule (Scaffidi et al., 2002). The released HMGB1 interacts with TLR4 on the antigen presenting cells to activate the antigen presenting cells (Yamazaki et al., 2014).
The Fas/FasL system is an essential mechanism to ensure T cell-mediated cytotoxicity. FasL binds to Fas, which is expressed on the cell surface, leading to apoptosis of Fas expressing cells. It has been shown that DDR deficiency causes increased expression of Fas on tumor cells (Jin et al., 2019), thereby promoting lymphocyte infiltration and anti-tumor immunity (Fig. 3b). RAP80 is a member of BRCA1-A complex with defined roles in regulating DNA damage repair and cell cycle checkpoints. Its expression level can be regulated by a USP13-mediated de-ubiquitination, which plays essential roles in regulating DDR (Li et al., 2017). The expression of RAP80 in breast cancer is lower than that those expressed in adjacent normal tissues, and its expression is related to tumor size and lymph node metastasis. Jin et al. reported that down-regulation of RAP80 can increase the expression of Fas, thus inducing cancer cell death (Jin et al., 2019). Fas protein expression has been shown to be up-regulated in dMMR tumors, which leads to increased sensitivity to FasL stimulation-induced tumor death (Raats et al., 2017).
Tumor aneuploid
Aneuploidy, a genetic alternation featured by the abnormal number of chromosomes, has been found to trigger the cellular stress, leading to local immune cell imbalance (Xian et al., 2021). In a pancancer analysis, DDR deficiency was found to be associated with the increased aneuploidy and the reduced infiltration of cytotoxic immune cells (Davoli et al., 2017), suggesting that aneuploidy may be negatively associated with immune cell infiltration. However, it is not always the case. For example, Thorsson et al. recently reported that aneuploidy positively correlates with tumor leukocyte fraction. Nonetheless, the composition of the aneuploid tumor microenvironment is dominated by macrophages that account for activation of tumor growth-factor-β (TGF-β) due to the immune suppressive phenotypes (Thorsson et al., 2018). Therefore, we can’t exclude the possibility that aneuploidy may inhibit anti-tumor immunity. Indeed, Auslander et al. reported recently that gastrointestinal and endometrial aneuploid tumors have the reduced mutational load, and therefore may show decreased response to immunotherapy (Auslander et al., 2020). These data suggested that aneuploidy is related to tumor antigenicity.
cGAS-STING
Cold tumors indicate a status that cancers that have not been recognized or have not provoked a strong response by the immune system. The cGAS-STING pathway is the major component in the innate immune system, and its activation is expected to be able to switch cold tumors to hot ones. Generally, the cGAS-STING pathway can be activated by mis-localized cytoplasmic DNA. As such, cGAS remains inactive and with low expression levels in cell. Whereas, in tumor cells, due to increased rates of replication, a large amount of DNA is retained in the cytoplasm, which increases the possibility of cGAS-STING pathway activation and increased anti-tumor immunity.
The key molecule to maintain cGAS signal immune balance is TREX1. As the main exonuclease in mammalian cells, it can degrade DNA and prevent over-activation of cGAS signaling (Diamond et al., 2018; Zhou et al., 2022). TREX1 dysfunction caused by gene mutation is associated with a variety of autoimmune diseases, such as Aicardi Goutières syndrome, systemic lupus erythematosus and retinal vascular disease (Pelzer et al., 2019; Xiao et al., 2019).
DDR deficiency can activate the cGAS-STING pathway, leading to increased anti-tumor immunity (Fig. 3c). For example, the cGAS-STING signaling pathway has been reported to be crucial for dMMR triggered anti-tumor immunity (Guan et al., 2021). The loss of MLH1 leads to the accumulation of cytoplasmic DNA in tumor cells, which increases the expression of IFNβ and T cell activation in a cGAS-STING dependent manner (Guan et al., 2021; Lu et al., 2021). The deficiency of additional key genes involved in DDR pathways, such as PARP1 (Chabanon et al., 2019; Shen et al., 2019), CHK1 (Sen et al., 2019), BRCA1 (Parkes et al., 2017), BRCA2 (Reisländer et al., 2019), ATM (Hu et al., 2021; Zhang et al., 2019), ATR (Sheng et al., 2020; Tang et al., 2021), Mre11 (Wardlaw & Petrini, 2022), NBSI (Abdisalaam et al., 2022) and ARID1A (Wang et al., 2020b) have been also shown to activate the cGAS-STING pathway in tumors. Furthermore, maintaining the stability of replication forks is very important for cells to cope with replication pressure and maintain genome stability. Abro1 or FANCD2 deficiency leads to the stagnation of replication fork degradation and the accumulation of cytoplasmic DNA, which activates the cGAS-SITNG signaling pathway and T cell immunity, dependently on the activation of DNA2 nuclease (Emam et al., 2022).
As mentioned above, DDR-regulated cGAS-STING pathway can promote immunogenicity. However, immuno-inhibitory effect can be produced under certain circumstances. For example, Bakhoum et al. reported that the chronic activation of cGAS-STING pathway is associated with the increased tumor metastasis (Bakhoum et al., 2018). Radiotherapy-stimulated STING pathway promotes tumor growth, which is mainly due to the increased infiltration of MDSCs (Liang et al., 2017). How to optimally and selectively activate the anti-tumor immunity of cGAS-STING warrants further exploration. In addition, despite being clear that the DDR defects-caused cytoplasmic DNA activates cGAS-STING, it remains obscure how cytoplasmic DNA is generated from the damaged nuclear genome. Furthermore, the molecular mechanism underlying translocation of genomic DNA fragments from the nucleus to the cytoplasm remains to be clarified. Moreover, it remains undefined about which form of chromatin DNA in the micronucleus contributes to the activation of cGAS.
Collectively, the above review has illustrated the close connection between DDR and cancer immunity. The combination of DDR targeted therapies and cancer immunotherapy is described in the following section.
DDR targets and cancer immunotherapy
Immunotherapy has revolutionized the treatment of cancer. However, immunotherapy response rates remain low in cancer patients in general (Hiam-Galvez et al., 2021). Of note, accumulating evidence suggest that DDR inhibitors, broadly used in clinical trials, can activate the immune system (Concannon et al., 2023). Such information warrants investigation into the combination of DDR inhibitors and immunotherapy to treat cancer. In this section, we will summarize DDR inhibitors used in cancer treatment, as well as their combinations with immunotherapy (Table 1).
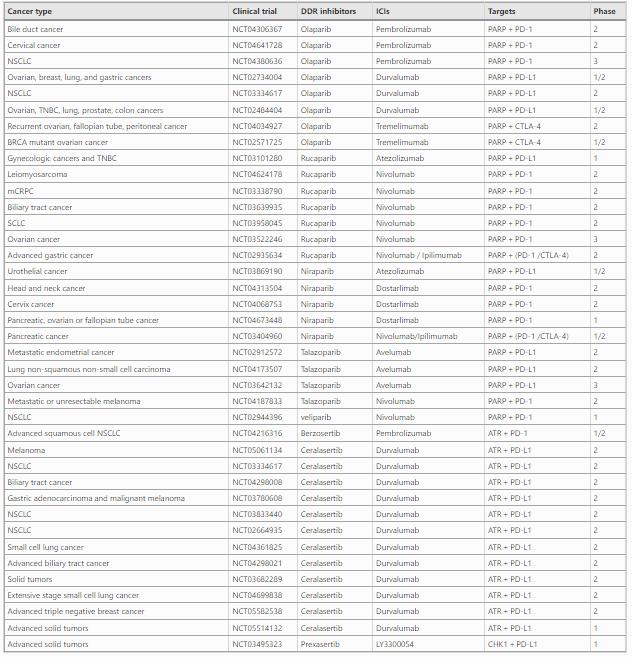
Table 1 Combinations of DDR inhibitors with ICIs
Parp inhibitors
The poly (ADP-ribose) polymerase (PARP) family plays a broad role in DNA repair, gene transcription and cell death (Richard et al., 2021). A number of PARP inhibitors (Slade, 2020), such as Olaparib, Rucaparib, Niraparib and Talazoparib, have been approved to treat cancers, such as breast cancer, pancreatic cancer, ovarian cancer, fallopian tube cancer and primary peritoneal cancer (Mateo et al., 2019), which is mainly based on the principle of synthetic lethality, the situation when mutation in either of two genes individually has no effect, but combining the mutations leads to death.
Due to the close association between DDR and immunity, combinations of PARP inhibitors and ICIs (anti-PD-L1, anti-PD-1, anti-CTLA-4) have been tested in clinical trials. According to the results from clinical trials (NCT02734004), combination of Olaparib and anti-PD-L1 (Durvalumab) showed promising antitumor activity and safety in BRCA-mutated metastatic breast cancer (Domchek et al., 2020). Similar positive therapeutic effects were reported in other tumors, such as ovarian, triple negative breast, lung, prostate and colorectal cancers (NCT02484404) (Karzai et al., 2018; Zimmer et al., 2019). Additionally, rucaparib, niraparib and talazoparib are also used in combination with ICIs (anti-PD-L1, anti-PD-1, anti-CTLA-4) to treat a variety of cancers, such as BRCA2 mutant ovarian cancer, drug-resistant ovarian cancer, and advanced pancreatic cancer (Konstantinopoulos et al., 2019; Reiss et al., 2022).
ATM and CHK2 inhibitors
ATM is primarily involved in the HRR pathway, as described above. Three ATM inhibitors are currently entering clinical trials: AZD-1390 (Durant et al., 2018), M-4076 (Fuchss et al., 2019), and XRD-0394 (Groelly et al., 2023). AZD-1390 can cross the blood–brain barrier, supporting its development for the treatment of glioblastoma multiforme as well as other brain malignancies (Jucaite et al., 2021). M4076 has just entered the clinical stage, and its pharmacological effects need to be further explored (trial number: NCT04882917). XRD-0394 is a dual inhibitor of ATM/DNA-PK for the treatment of metastatic, locally advanced, or recurrent solid tumors (trial number: NCT05002140). In addition, there are few specific inhibitors for the ATM downstream effector CHK2. For example, prexasertib is an effective CHK1/CHK2 inhibitor, which has entered phase II of cancer related clinical trials (Lee et al., 2018). Recently, many studies have investigated the effect of ATM and CHK2 inhibitors on anti-tumor immunity. The results demonstrated that the ATM and CHK2 are promising targets to improve immune checkpoint blockade therapy (Wang et al., 2020). In addition, inhibition of the ATM/CHK2 axis activates cGAS-STING, and thus increases tumor-infiltrating lymphocytes (Wang et al., 2020). Therefore, the combination of an ATM/CHK2 inhibitor and immune therapy has great therapeutic potential.
ATR and CHK1 inhibitors
ATR is one of the core kinases of DDR with CHK1 as its substrate. ATR inhibitors have been widely explored in combination with ICIs, such as anti-PD-1/PD-L1. For example, a phase II trial evaluated the efficiency of the ATR inhibitor AZD6738, plus anti-PD-L1 durvalumab in patients with advanced/metastatic melanoma, which shows promising synergistic antitumor activity (Kim et al., 2022). Another phase II clinical trial (NCT03780608) showed that AZD6738 combined with anti-PD-L1 had antitumor activity for advanced gastric cancer (Kwon et al., 2022). In agreement, AZD6738 was found to enhance the activity of CD8 + T cells in a mouse model (Sheng et al., 2020). Further mechanistic studies reveal that AZD6738 decreased PD-L1 expression and markedly decreased the numbers of tumor-infiltrating T-regs. Furthermore, CHK1 inhibitors, Prexasertib, is also undergoing clinical trial tests. Recently, prexasertib in combination with anti-PD-L1 antibody LY3300054 was tolerable and demonstrated preliminary activity in high-grade serous ovarian cancer with evidence of cytotoxic T-cell activation in patient blood samples (NCT03495323) (Do et al., 2021).
DNAPK inhibitors
DNAPK is an initiator kinase involved in the NHEJ repair cascade. DNAPK inhibitor Nedisertib have entered phase I/II clinical trials (Carr et al., 2022). DNAPK inhibitors significantly reduces the expression of PD-1 in T cells and increases granzyme B expression in NK-cells (Nakamura et al., 2021a). Additionally, Carr et al. reported that the DNAPK inhibitor, Nedisertib, activates the STING-dependent pathway, suggesting involvement of the innate immunity in DNAPK regulated DNA repair signaling (Carr et al., 2022). It has become clear that DNAPK inhibitor treatment can modulate both the innate and adaptive immune systems, providing important insights into new combined strategies for the treatment of cancer. Of note, additional DNA-PKcs inhibitors such as NU7441, CC115 and AZD7648, have been developed (Liang et al., 2022). Their clinical effects and the possible involvement of cancer immunity, as well as the combination approaches, warrant additional investigation.
In addition to the above mentioned, more clinical trials of the combination of DDR inhibitors and immunotherapy are under way, which bring more opportunities for cancer treatment (Table 1).
Remarks
The close association between DDR and cancer has been recognized for a long time. DDR represents a promising target for cancer therapies, including both chemoradiotherapies and targeted therapies. With our increasing understanding of cancer biology, immune evasion has attracted significant attention, which has brought about a new treatment approach against cancer. A number of immune therapeutic drugs have been approved to treat advanced solid tumors, however, only 20–30% of patients currently benefit from such treatments. Combined therapies thus can be further developed to improve treatment efficiency. Recently, an increasing body of work suggests DDR broadly cross-talks with anti-tumor immune responses in multiple ways. This has led to combinations of DDR modulation and immune therapies being tested in clinic, which has brought clinical benefits at least for some patients. However, further basic research and clinical trials are needed to explore the mechanisms and safety of DDR inhibitor and ICIs as combined therapies.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abbasi, S., Parmar, G., Kelly, R. D., Balasuriya, N., & Schild-Poulter, C. (2021). The Ku complex: Recent advances and emerging roles outside of non-homologous end-joining. Cellular and Molecular Life Sciences, 78(10), 4589–4613.
Abbotts, R., & Madhusudan, S. (2010). Human AP endonuclease 1 (APE1): from mechanistic insights to druggable target in cancer. Cancer Treatment Reviews, 36(5), 425–435.
Abdisalaam, S., Mukherjee, S., Bhattacharya, S., Kumari, S., Sinha, D., Ortega, J., Li, G. M., Sadek, H. A., Krishnan, S., & Asaithamby, A. (2022). NBS1-CtIP-mediated DNA end resection suppresses cGAS binding to micronuclei. Nucleic Acids Research, 50(5), 2681–2699.
Ablasser, A., & Chen, Z. J. (2019). cGAS in action: Expanding roles in immunity and inflammation. Science, 363(6431), eaat8657.
Auslander, N., Wolf, Y. I., & Koonin, E. V. (2020). Interplay between DNA damage repair and apoptosis shapes cancer evolution through aneuploidy and microsatellite instability. Nature Communications, 11(1), 1234.
Bakhoum, S. F., Ngo, B., Laughney, A. M., Cavallo, J. A., Murphy, C. J., Ly, P., Shah, P., Sriram, R. K., Watkins, T. B. K., Taunk, N. K., Duran, M., Pauli, C., Shaw, C., Chadalavada, K., Rajasekhar, V. K., Genovese, G., Venkatesan, S., Birkbak, N. J., & McGranahanCantley, N. L. C. (2018). Chromosomal instability drives metastasis through a cytosolic DNA response. Nature, 553(7689), 467–472.
Baretti, M., & Le, D. T. (2018). DNA mismatch repair in cancer. Pharmacology & Therapeutics, 189, 45–62.
Batenburg, N. L., Walker, J. R., Coulombe, Y., Sherker, A., Masson, J. Y., & Zhu, X. D. (2019). CSB interacts with BRCA1 in late S/G2 to promote MRN- and CtIP-mediated DNA end resection. Nucleic Acids Research, 47(20), 10678–10692.
Bauer, N. C., Corbett, A. H., & Doetsch, P. W. (2015). The current state of eukaryotic DNA base damage and repair. Nucleic Acids Research, 43(21), 10083–10101.
Beard, W. A., Horton, J. K., Prasad, R., & Wilson, S. H. (2019). Eukaryotic base excision repair: New approaches shine light on mechanism. Annual Review of Biochemistry, 88, 137–162.
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Arain, M. A., Chen, Y. J., Ciombor, K. K., Cohen, S., Cooper, H. S., Deming, D., Farkas, L., Garrido-Laguna, I., Grem, J. L., Gunn, A., Hecht, J. R., Hoffe, S., Hubbard, J., Hunt, S., Johung, K. L., Kirilcuk, N., & Gurski, L. A. (2021). Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 19(3), 329–359.
Berndt, S. I., Huang, W. Y., Fallin, M. D., Helzlsouer, K. J., Platz, E. A., Weissfeld, J. L., Church, T. R., Welch, R., Chanock, S. J., & Hayes, R. B. (2007). Genetic variation in base excision repair genes and the prevalence of advanced colorectal adenoma. Cancer Research, 67(3), 1395–1404.
Bhat, K. P., & Cortez, D. (2018). RPA and RAD51: Fork reversal, fork protection, and genome stability. Nature Structural & Molecular Biology, 25(6), 446–453.
Boland, C. R., & Goel, A. (2010). Microsatellite instability in colorectal cancer. Gastroenterology, 138(6), 2073-2087.e3.
Bonneville, R., Krook, M. A., Kautto, E. A., Miya, J., Wing, M. R., Chen, H. Z., Reeser, J. W., Yu, L., & Roychowdhury, S. (2017). Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol, 1, PO.17.00073.
Botta, G. P., Kato, S., Patel, H., Fanta, P., Lee, S., Okamura, R., & Kurzrock, R. (2021). SWI/SNF complex alterations as a biomarker of immunotherapy efficacy in pancreatic cancer. JCI Insight, 6(18), e150453.
Boulton, S. J., & Jackson, S. P. (1996). Saccharomyces cerevisiae Ku70 potentiates illegitimate DNA double-strand break repair and serves as a barrier to error-prone DNA repair pathways. EMBO Journal, 15(18), 5093–5103.
Bradford, K. C., Wilkins, H., Hao, P., Li, Z. M., Wang, B., Burke, D., Wu, D., Smith, A. E., Spaller, L., Du, C., Gauer, J. W., Chan, E., Hsieh, P., Weninger, K. R., & Erie, D. A. (2020). Dynamic human MutSα-MutLα complexes compact mismatched DNA. Proceedings of the National Academy of Sciences U S A, 117(28), 16302–16312.
Brady, N., Gaymes, T. J., Cheung, M., Mufti, G. J., & Rassool, F. V. (2003). Increased error-prone NHEJ activity in myeloid leukemias is associated with DNA damage at sites that recruit key nonhomologous end-joining proteins. Cancer Research, 63(8), 1798–1805.
Brueckner, F., Hennecke, U., Carell, T., & Cramer, P. (2007). CPD damage recognition by transcribing RNA polymerase II. Science, 315(5813), 859–862.
Burger, M., Denzinger, S., Hammerschmied, C. G., Tannapfel, A., Obermann, E. C., Wieland, W. F., Hartmann, A., & Stoehr, R. (2006). Elevated microsatellite alterations at selected tetranucleotides (EMAST) and mismatch repair gene expression in prostate cancer. Journal of Molecular Medicine (berlin, Germany), 84(10), 833–841.
Carr, M. I., Chiu, L. Y., Guo, Y., Xu, C., Lazorchak, A. S., Yu, H., Qin, G., Qi, J., Marelli, B., Lan, Y., Sun, Q., Czauderna, F., Zenke, F. T., Blaukat, A., & Vassilev, L. T. (2022). DNA-PK inhibitor peposertib amplifies radiation-induced inflammatory micronucleation and enhances TGFβ/PD-L1 targeted cancer immunotherapy. Molecular Cancer Research, 20(4), 568–582.
Chabanon, R. M., Muirhead, G., Krastev, D. B., Adam, J., Morel, D., Garrido, M., Lamb, A., Hénon, C., Dorvault, N., Rouanne, M., Marlow, R., Bajrami, I., Cardeñosa, M. L., Konde, A., Besse, B., Ashworth, A., Pettitt, S. J., Haider, S., & MarabellePostel-Vinay, A. S. (2019). PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. The Journal of Clinical Investigation, 129(3), 1211–1228.
Chabanon, R. M., Rouanne, M., Lord, C. J., Soria, J. C., Pasero, P., & Postel-Vinay, S. (2021). Targeting the DNA damage response in immuno-oncology: Developments and opportunities. Nature Reviews Cancer, 21(11), 701–717.
Chibaya, L., Karim, B., Zhang, H., & Jones, S. N. (2021). Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proceedings of the National Academy of Sciences USA, 118(4), e2003193118.
Concannon, K., Morris, B. B., Gay, C. M., & Byers, L. A. (2023). Combining targeted DNA repair inhibition and immune-oncology approaches for enhanced tumor control. Molecular Cell, 83(5), 660–680.
Connor, A. A., Denroche, R. E., Jang, G. H., Timms, L., Kalimuthu, S. N., Selander, I., McPherson, T., Wilson, G. W., Chan-Seng-Yue, M. A., Borozan, I., Ferretti, V., Grant, R. C., Lungu, I. M., Costello, E., Greenhalf, W., Palmer, D., Ghaneh, P., Neoptolemos, J. P., & BuchlerGallinger, M. S. (2017). Association of distinct mutational signatures with correlates of increased immune activity in pancreatic ductal adenocarcinoma. JAMA Oncology, 3(6), 774–783.
Constantin, N., Dzantiev, L., Kadyrov, F. A., & Modrich, P. (2005). Human mismatch repair: Reconstitution of a nick-directed bidirectional reaction. Journal of Biological Chemistry, 280(48), 39752–39761.
Cortes-Ciriano, I., Lee, S., Park, W. Y., Kim, T. M., & Park, P. J. (2017). A molecular portrait of microsatellite instability across multiple cancers. Nature Communications, 8, 15180.
da Costa, A. A. B. A., Chowdhury, D., Shapiro, G. I., D’Andrea, A. D., & Konstantinopoulos, P. A. (2023). Targeting replication stress in cancer therapy. Nature Reviews Drug Discovery, 22(1), 38–58.
Daley, J. M., Gaines, W. A., Kwon, Y., & Sung, P. (2014). Regulation of DNA pairing in homologous recombination. Cold Spring Harbor Perspectives in Biology, 6(11), a017954.
Das, A., Sudhaman, S., Morgenstern, D., Coblentz, A., Chung, J., Stone, S. C., Alsafwani, N., Liu, Z. A., Karsaneh, O. A. A., Soleimani, S., Ladany, H., Chen, D., Zatzman, M., Cabric, V., Nobre, L., Bianchi, V., Edwards, M., Sambira Nahum, L. C., Ercan, A. B., & Tabori, U. (2022). Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nature Medicine, 28(1), 125–135.
Davoli, T., Uno, H., Wooten, E. C., & Elledge, S. J. (2017). Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science, 355(6322), eaaf8399.
Demin, A. A., Hirota, K., Tsuda, M., Adamowicz, M., Hailstone, R., Brazina, J., Gittens, W., Kalasova, I., Shao, Z., Zha, S., Sasanuma, H., Hanzlikova, H., Takeda, S., & Caldecott, K. W. (2021). XRCC1 prevents toxic PARP1 trapping during DNA base excision repair. Molecular Cell, 81(14), 3018-3030.e5.
Diamond, J. M., Vanpouille-Box, C., Spada, S., Rudqvist, N. P., Chapman, J. R., Ueberheide, B. M., Pilones, K. A., Sarfraz, Y., Formenti, S. C., & Demaria, S. (2018). Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunology Research, 6(8), 910–920.
Do, K. T., Manuszak, C., Thrash, E., Giobbie-Hurder, A., Hu, J., Kelland, S., Powers, A., de Jonge, A., Shapiro, G. I., & Severgnini, M. (2021). Immune modulating activity of the CHK1 inhibitor prexasertib and anti-PD-L1 antibody LY3300054 in patients with high-grade serous ovarian cancer and other solid tumors. Cancer Immunology, Immunotherapy, 70(10), 2991–3000.
Domchek, S. M., Postel-Vinay, S., Im, S. A., Park, Y. H., Delord, J. P., Italiano, A., Alexandre, J., You, B., Bastian, S., Krebs, M. G., Wang, D., Waqar, S. N., Lanasa, M., Rhee, J., Gao, H., Rocher-Ros, V., Jones, E. V., Gulati, S., & Coenen-StassKaufman, A. B. (2020). Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. The Lancet Oncology, 21(9), 1155–1164.
Downs, J. A., & Jackson, S. P. (2004). A means to a DNA end: The many roles of Ku. Nature Reviews Molecular Cell Biology, 5(5), 367–378.
Durant, S. T., Zheng, L., Wang, Y., Chen, K., Zhang, L., Zhang, T., Yang, Z., Riches, L., Trinidad, A. G., Fok, J. H. L., Hunt, T., Pike, K. G., Wilson, J., Smith, A., Colclough, N., Reddy, V. P., Sykes, A., Janefeldt, A., Johnström, P., & Pass, M. (2018). The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Science Advances, 4(6), eaat1719.
Emam, A., Wu, X., Xu, S., Wang, L., Liu, S., & Wang, B. (2022). Stalled replication fork protection limits cGAS-STING and P-body-dependent innate immune signalling. Nature Cell Biology, 24(7), 1154–1164.
Feng, M., Zhao, Z., Yang, M., Ji, J., & Zhu, D. (2021). T-cell-based immunotherapy in colorectal cancer. Cancer Letters, 498, 201–209.
Foo, T. K., Vincelli, G., Huselid, E., Her, J., Zheng, H., Simhadri, S., Wang, M., Huo, Y., Li, T., Yu, X., Li, H., Zhao, W., Bunting, S. F., & Xia, B. (2021). ATR/ATM-mediated phosphorylation of BRCA1 T1394 promotes homologous recombinational repair and G(2)-M checkpoint maintenance. Cancer Research, 81(18), 4676–4684.
Fousteri, M., & Mullenders, L. H. (2008). Transcription-coupled nucleotide excision repair in mammalian cells: Molecular mechanisms and biological effects. Cell Research, 18(1), 73–84.
Friedberg, E. C. (2008). A brief history of the DNA repair field. Cell Research, 18(1), 3–7.
Fuchss, T., Graedler, U., Schiemann, K., Kuhn, D., Kubas, H., Dahmen, H., Zimmermann, A., Zenke, F., & Blaukat, A. (2019). Abstract 3500: Highly potent and selective ATM kinase inhibitor M4076: A clinical candidate drug with strong anti-tumor activity in combination therapies. Cancer Research, 79(13_Supplement), 3500–3500.
Furgason, J. M., & el Bahassi, M. (2013). Targeting DNA repair mechanisms in cancer. Pharmacology & Therapeutics, 137(3), 298–308.
Gasser, S., & Raulet, D. (2006). The DNA damage response, immunity and cancer. Seminars in Cancer Biology, 16(5), 344–347.
Gell, D., & Jackson, S. P. (1999). Mapping of protein-protein interactions within the DNA-dependent protein kinase complex. Nucleic Acids Research, 27(17), 3494–3502.
Geng, A., Tang, H., Huang, J., Qian, Z., Qin, N., Yao, Y., Xu, Z., Chen, H., Lan, L., Xie, H., Zhang, J., Jiang, Y., & Mao, Z. (2020). The deacetylase SIRT6 promotes the repair of UV-induced DNA damage by targeting DDB2. Nucleic Acids Research, 48(16), 9181–9194.
Genschel, J., & Modrich, P. (2009). Functions of MutLalpha, replication protein A (RPA), and HMGB1 in 5’-directed mismatch repair. Journal of Biological Chemistry, 284(32), 21536–21544.
Grant, R. C., Denroche, R., Jang, G. H., Nowak, K. M., Zhang, A., Borgida, A., Holter, S., Topham, J. T., Wilson, J., Dodd, A., Jang, R., Prince, R., Karasinska, J. M., Schaeffer, D. F., Wang, Y., Zogopoulos, G., Berry, S., Simeone, D., Renouf, D. J., & Gallinger, S. (2021). Clinical and genomic characterisation of mismatch repair deficient pancreatic adenocarcinoma. Gut, 70(10), 1894–1903.
Gregersen, L. H., & Svejstrup, J. Q. (2018). The cellular response to transcription-blocking DNA damage. Trends in Biochemical Sciences, 43(5), 327–341.
Groelly, F. J., Fawkes, M., Dagg, R. A., Blackford, A. N., & Tarsounas, M. (2023). Targeting DNA damage response pathways in cancer. Nature Reviews Cancer, 23(2), 78–94.
Guan, J., Lu, C., Jin, Q., Lu, H., Chen, X., Tian, L., Zhang, Y., Ortega, J., Zhang, J., Siteni, S., Chen, M., Gu, L., Shay, J. W., Davis, A. J., Chen, Z. J., Fu, Y. X., & Li, G. M. (2021). MLH1 Deficiency-triggered DNA hyperexcision by exonuclease 1 activates the cGAS-STING pathway. Cancer Cell, 39(1), 109-121.e5.
Haahr, P., Hoffmann, S., Tollenaere, M. A., Ho, T., Toledo, L. I., Mann, M., Bekker-Jensen, S., Räschle, M., & Mailand, N. (2016). Activation of the ATR kinase by the RPA-binding protein ETAA1. Nature Cell Biology, 18(11), 1196–1207.
He, X., & Xu, C. (2020). Immune checkpoint signaling and cancer immunotherapy. Cell Research, 30(8), 660–669.
Hiam-Galvez, K. J., Allen, B. M., & Spitzer, M. H. (2021). Systemic immunity in cancer. Nature Reviews Cancer, 21(6), 345–359.
Hu, M., Zhou, M., Bao, X., Pan, D., Jiao, M., Liu, X., Li, F., & Li, C. Y. (2021). ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. Journal of Clinical Investigation, 131(3), e139333.
Huang, F., Tanaka, H., Knudsen, B. S., & Rutgers, J. K. (2020). Mutant POLQ and POLZ/REV3L DNA polymerases may contribute to the favorable survival of patients with tumors with POLE mutations outside the exonuclease domain. BMC Medical Genetics, 21(1), 167.
Huang, R., & Zhou, P. K. (2021). DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduction and Targeted Therapy, 6(1), 254.
Hura, G. L., Tsai, C. L., Claridge, S. A., Mendillo, M. L., Smith, J. M., Williams, G. J., Mastroianni, A. J., Alivisatos, A. P., Putnam, C. D., Kolodner, R. D., & Tainer, J. A. (2013). DNA conformations in mismatch repair probed in solution by X-ray scattering from gold nanocrystals. Proceedings of the National Academy of Sciences U S A, 110(43), 17308–17313.
Jackson, S. P., & Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature, 461(7267), 1071–1078.
Jager, M., Blokzijl, F., Kuijk, E., Bertl, J., Vougioukalaki, M., Janssen, R., Besselink, N., Boymans, S., de Ligt, J., Pedersen, J. S., Hoeijmakers, J., Pothof, J., van Boxtel, R., & Cuppen, E. (2019). Deficiency of nucleotide excision repair is associated with mutational signature observed in cancer. Genome Research, 29(7), 1067–1077.
Jayaram, S., Ketner, G., Adachi, N., & Hanakahi, L. A. (2008). Loss of DNA ligase IV prevents recognition of DNA by double-strand break repair proteins XRCC4 and XLF. Nucleic Acids Research, 36(18), 5773–5786.
Jhunjhunwala, S., Hammer, C., & Delamarre, L. (2021). Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nature Reviews Cancer, 21(5), 298–312.
Jiang, M., Jia, K., Wang, L., Li, W., Chen, B., Liu, Y., Wang, H., Zhao, S., He, Y., & Zhou, C. (2021). Alterations of DNA damage response pathway: Biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharmaceutica Sinica B, 11(10), 2983–2994.
Jiang, W., Crowe, J. L., Liu, X., Nakajima, S., Wang, Y., Li, C., Lee, B. J., Dubois, R. L., Liu, C., Yu, X., Lan, L., & Zha, S. (2015). Differential phosphorylation of DNA-PKcs regulates the interplay between end-processing and end-ligation during nonhomologous end-joining. Molecular Cell, 58(1), 172–185.
Jin, G., Mao, X., Qiao, Z., Chen, B., & Jin, F. (2019). RAP80 expression in breast cancer and its relationship with apoptosis in breast cancer cells. Oncotargets and Therapy, 12, 625–634.
Jucaite, A., Stenkrona, P., Cselényi, Z., De Vita, S., Buil-Bruna, N., Varnäs, K., Savage, A., Varrone, A., Johnström, P., Schou, M., Davison, C., Sykes, A., Pilla Reddy, V., Hoch, M., Vazquez-Romero, A., Moein, M. M., Halldin, C., Merchant, M. S., & PassFarde, M. L. (2021). Brain exposure of the ATM inhibitor AZD1390 in humans-a positron emission tomography study. Neuro-Oncology, 23(4), 687–696.
Kang, M. S., Ryu, E., Lee, S. W., Park, J., Ha, N. Y., Ra, J. S., Kim, Y. J., Kim, J., Abdel-Rahman, M., Park, S. H., Lee, K. Y., Kim, H., Kang, S., & Myung, K. (2019). Regulation of PCNA cycling on replicating DNA by RFC and RFC-like complexes. Nature Communications, 10(1), 2420.
Karzai, F., VanderWeele, D., Madan, R. A., Owens, H., Cordes, L. M., Hankin, A., Couvillon, A., Nichols, E., Bilusic, M., Beshiri, M. L., Kelly, K., Krishnasamy, V., Lee, S., Lee, M. J., Yuno, A., Trepel, J. B., Merino, M. J., Dittamore, R., & MartéDahut, J. W. L. (2018). Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. Journal for Immunotherapy of Cancer, 6(1), 141.
Kijas, A. W., Lim, Y. C., Bolderson, E., Cerosaletti, K., Gatei, M., Jakob, B., Tobias, F., Taucher-Scholz, G., Gueven, N., Oakley, G., Concannon, P., Wolvetang, E., Khanna, K. K., Wiesmüller, L., & Lavin, M. F. (2015). ATM-dependent phosphorylation of MRE11 controls extent of resection during homology directed repair by signalling through Exonuclease 1. Nucleic Acids Research, 43(17), 8352–8367.
Kim, R., Kwon, M., An, M., Kim, S. T., Smith, S. A., Loembé, A. B., Mortimer, P. G. S., Armenia, J., Lukashchuk, N., Shah, N., Dean, E., Park, W. Y., & Lee, J. (2022). Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced/metastatic melanoma who have failed prior anti-PD-1 therapy. Annals of Oncology, 33(2), 193–203.
Konstantinopoulos, P. A., Waggoner, S., Vidal, G. A., Mita, M., Moroney, J. W., Holloway, R., Van Le, L., Sachdev, J. C., Chapman-Davis, E., Colon-Otero, G., Penson, R. T., Matulonis, U. A., Kim, Y. B., Moore, K. N., Swisher, E. M., Färkkilä, A., D’Andrea, A., Stringer-Reasor, E., & WangMunster, J. P. (2019). Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncology, 5(8), 1141–1149.
Kroemer, G., Galassi, C., Zitvogel, L., & Galluzzi, L. (2022). Immunogenic cell stress and death. Nature Immunology, 23(4), 487–500.
Kroemer, G., Galluzzi, L., Kepp, O., & Zitvogel, L. (2013). Immunogenic cell death in cancer therapy. Annual Review of Immunology, 31(1), 51–72.
Kunkel, T. A., & Erie, D. A. (2015). Eukaryotic mismatch repair in relation to DNA replication. Annual Review of Genetics, 49, 291–313.
Kwon, M., Kim, G., Kim, R., Kim, K. T., Kim, S. T., Smith, S., Mortimer, P. G. S., Hong, J. Y., Loembé, A. B., Irurzun-Arana, I., Koulai, L., Kim, K. M., Kang, W. K., Dean, E., Park, W. Y., & Lee, J. (2022). Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced gastric cancer. Journal for ImmunoTherapy of Cancer, 10(7), 504.
Le, D. T., Durham, J. N., Smith, K. N., Wang, H., Bartlett, B. R., Aulakh, L. K., Lu, S., Kemberling, H., Wilt, C., Luber, B. S., Wong, F., Azad, N. S., Rucki, A. A., Laheru, D., Donehower, R., Zaheer, A., Fisher, G. A., Crocenzi, T. S., Lee, J. J., & Diaz Jr., L. A. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science, 357(6349), 409–413.
Lee, J. M., Nair, J., Zimmer, A., Lipkowitz, S., Annunziata, C. M., Merino, M. J., Swisher, E. M., Harrell, M. I., Trepel, J. B., Lee, M. J., Bagheri, M. H., Botesteanu, D. A., Steinberg, S. M., Minasian, L., Ekwede, I., & Kohn, E. C. (2018). Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: A first-in-class proof-of-concept phase 2 study. The Lancet Oncology, 19(2), 207–215.
Lee, J. H., & Paull, T. T. (2004). Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science, 304(5667), 93–96.
Leitner-Dagan, Y., Sevilya, Z., Pinchev, M., Kramer, R., Elinger, D., Roisman, L. C., Rennert, H. S., Schechtman, E., Freedman, L., Rennert, G., Livneh, Z., & Paz-Elizur, T. (2012). N-methylpurine DNA glycosylase and OGG1 DNA repair activities: Opposite associations with lung cancer risk. Journal of the National Cancer Institute, 104(22), 1765–1769.
Li, W., Amei, A., Bui, F., Norouzifar, S., Lu, L., & Wang, Z. (2021). Impact of neoantigen expression and T-cell activation on breast cancer survival. Cancers (basel), 13(12), 2879.
Li, Y., Luo, K., Yin, Y., Wu, C., Deng, M., Li, L., Chen, Y., Nowsheen, S., Lou, Z., & Yuan, J. (2017). USP13 regulates the RAP80-BRCA1 complex dependent DNA damage response. Nature Communications, 8, 15752.
Liang, H., Deng, L., Hou, Y., Meng, X., Huang, X., Rao, E., Zheng, W., Mauceri, H., Mack, M., Xu, M., Fu, Y. X., & Weichselbaum, R. R. (2017). Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nature Communications, 8(1), 1736.
Liang, S., Thomas, S. E., Chaplin, A. K., Hardwick, S. W., Chirgadze, D. Y., & Blundell, T. L. (2022). Structural insights into inhibitor regulation of the DNA repair protein DNA-PKcs. Nature, 601(7894), 643–648.
Liang, Z., Kumar, V., Le Bouteiller, M., Zurita, J., Kenrick, J., Lin, S. G., Lou, J., Hu, J., Ye, A. Y., Boboila, C., Alt, F. W., & Frock, R. L. (2021). Ku70 suppresses alternative end joining in G1-arrested progenitor B cells. Proceedings of the National Academy of Sciences USA, 118(21), e2103630118.
Lieber, M. R. (2023). Pol X DNA polymerases contribute to NHEJ flexibility. Nature Structural & Molecular Biology, 30(1), 5–8.
Lin, Y., Raj, J., Li, J., Ha, A., Hossain, M. A., Richardson, C., Mukherjee, P., & Yan, S. (2020). APE1 senses DNA single-strand breaks for repair and signaling. Nucleic Acids Research, 48(4), 1925–1940.
Liu, S., Gӧnen, M., Stadler, Z. K., Weiser, M. R., Hechtman, J. F., Vakiani, E., Wang, T., Vyas, M., Joneja, U., Al-Bayati, M., Segal, N. H., Smith, J. J., King, S., Guercio, S., Ntiamoah, P., Markowitz, A. J., Zhang, L., Cercek, A., & Garcia-AguilarShia, J. J. (2019). Cellular localization of PD-L1 expression in mismatch-repair-deficient and proficient colorectal carcinomas. Modern Pathology, 32(1), 110–121.
Lizardo, D. Y., Kuang, C., Hao, S., Yu, J., Huang, Y., & Zhang, L. (2020). Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochimica Et Biophysica Acta-Reviews on Cancer, 1874(2), 188447.
Lord, C. J., & Ashworth, A. (2012). The DNA damage response and cancer therapy. Nature, 481(7381), 287–294.
Lu, C., Guan, J., Lu, S., Jin, Q., Rousseau, B., Lu, T., Stephens, D., Zhang, H., Zhu, J., Yang, M., Ren, Z., Liang, Y., Liu, Z., Han, C., Liu, L., Cao, X., Zhang, A., Qiao, J., & BattenFu, K. Y. X. (2021). DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell, 39(1), 96-108.e6.
Ma, X., Riaz, N., Samstein, R. M., Lee, M., Makarov, V., Valero, C., Chowell, D., Kuo, F., Hoen, D., Fitzgerald, C. W. R., Jiang, H., Alektiar, J., Alban, T. J., Juric, I., Parthasarathy, P. B., Zhao, Y., Sabio, E. Y., Verma, R., Srivastava, R. M., & MChan, T. A. (2022). Functional landscapes of POLE and POLD1 mutations in checkpoint blockade-dependent antitumor immunity. Nature Genetics, 54(7), 996–1012.
Ma, Y., Pannicke, U., Schwarz, K., & Lieber, M. R. (2002). Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell, 108(6), 781–794.
Marabelle, A., Le, D. T., Ascierto, P. A., Di Giacomo, A. M., De Jesus-Acosta, A., Delord, J. P., Geva, R., Gottfried, M., Penel, N., Hansen, A. R., Piha-Paul, S. A., Doi, T., Gao, B., Chung, H. C., Lopez-Martin, J., Bang, Y. J., Frommer, R. S., Shah, M., & GhoriDiaz Jr., R. L. A. (2020). Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the Phase II KEYNOTE-158 Study. Journal of Clinical Oncology, 38(1), 1–10.
Mardis, E. R. (2019). Neoantigens and genome instability: Impact on immunogenomic phenotypes and immunotherapy response. Genome Med, 11(1), 71.
Mateo, J., Lord, C. J., Serra, V., Tutt, A., Balmaña, J., Castroviejo-Bermejo, M., Cruz, C., Oaknin, A., Kaye, S. B., & de Bono, J. S. (2019). A decade of clinical development of PARP inhibitors in perspective. Annals of Oncology, 30(9), 1437–1447.
Matsumoto, S., Cavadini, S., Bunker, R. D., Grand, R. S., Potenza, A., Rabl, J., Yamamoto, J., Schenk, A. D., Schübeler, D., Iwai, S., Sugasawa, K., Kurumizaka, H., & Thomä, N. H. (2019). DNA damage detection in nucleosomes involves DNA register shifting. Nature, 571(7763), 79–84.
McAlpine, J. N., Porter, H., Köbel, M., Nelson, B. H., Prentice, L. M., Kalloger, S. E., Senz, J., Milne, K., Ding, J., Shah, S. P., Huntsman, D. G., & Gilks, C. B. (2012). BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Modern Pathology, 25(5), 740–750.
Mehrvarz Sarshekeh, A., Alshenaifi, J., Roszik, J., Manyam, G. C., Advani, S. M., Katkhuda, R., Verma, A., Lam, M., Willis, J., Shen, J. P., Morris, J., Davis, J. S., Loree, J. M., Lee, H. M., Ajani, J. A., Maru, D. M., Overman, M. J., & Kopetz, S. (2021). ARID1A mutation may define an immunologically active subgroup in patients with microsatellite stable colorectal cancer. Clinical Cancer Research, 27(6), 1663–1670.
Mendillo, M. L., Putnam, C. D., Mo, A. O., Jamison, J. W., Li, S., Woods, V. L., Jr., & Kolodner, R. D. (2010). Probing DNA- and ATP-mediated conformational changes in the MutS family of mispair recognition proteins using deuterium exchange mass spectrometry. Journal of Biological Chemistry, 285(17), 13170–13182.
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S., Liu, Q., Cochran, C., Bennett, L. M., Ding, W., et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science, 266(5182), 66–71.
Mullins, E. A., Rodriguez, A. A., Bradley, N. P., & Eichman, B. F. (2019). Emerging roles of DNA glycosylases and the base excision repair pathway. Trends in Biochemical Sciences, 44(9), 765–781.
Murphy, M. A., & Wentzensen, N. (2011). Frequency of mismatch repair deficiency in ovarian cancer: A systematic review This article is a US Government work and as such, is in the public domain of the United States of America. International Journal of Cancer, 129(8), 1914–1922.
Nakamura, K., Karmokar, A., Farrington, P. M., James, N. H., Ramos-Montoya, A., Bickerton, S. J., Hughes, G. D., Illidge, T. M., Cadogan, E. B., Davies, B. R., Dovedi, S. J., & Valge-Archer, V. (2021a). Inhibition of DNA-PK with AZD7648 sensitizes tumor cells to radiotherapy and induces type I IFN-dependent durable tumor control. Clinical Cancer Research, 27(15), 4353–4366.
Nakamura, T., Okabe, K., Hirayama, S., Chirifu, M., Ikemizu, S., Morioka, H., Nakabeppu, Y., & Yamagata, Y. (2021b). Structure of the mammalian adenine DNA glycosylase MUTYH: Insights into the base excision repair pathway and cancer. Nucleic Acids Research, 49(12), 7154–7163.
Nakamura, Y. (1998). ATM: The p53 booster. Nature Medicine, 4(11), 1231–1232.
Nakazawa, Y., Hara, Y., Oka, Y., Komine, O., van den Heuvel, D., Guo, C., Daigaku, Y., Isono, M., He, Y., Shimada, M., Kato, K., Jia, N., Hashimoto, S., Kotani, Y., Miyoshi, Y., Tanaka, M., Sobue, A., Mitsutake, N., & SuganamiOgi, T. T. (2020). Ubiquitination of DNA damage-stalled RNAPII promotes transcription-coupled repair. Cell, 180(6), 1228-1244.e24.
Nguyen, L., Martens, J. W. M., Van Hoeck, A., & Cuppen, E. (2020). Pan-cancer landscape of homologous recombination deficiency. Nature Communications, 11(1), 5584.
Ni, M., Zhang, W. Z., Qiu, J. R., Liu, F., Li, M., Zhang, Y. J., Liu, Q., & Bai, J. (2014). Association of ERCC1 and ERCC2 polymorphisms with colorectal cancer risk in a Chinese population. Science and Reports, 4, 4112.
Nolan, E., Savas, P., Policheni, A. N., Darcy, P. K., Vaillant, F., Mintoff, C. P., Dushyanthen, S., Mansour, M., Pang, J. B., Fox, S. B., Perou, C. M., Visvader, J. E., Gray, D. H. D., Loi, S., & Lindeman, G. J. (2017). Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Science Translational Medicine, 9(393), eaal4922.
Overman, M. J., McDermott, R., Leach, J. L., Lonardi, S., Lenz, H. J., Morse, M. A., Desai, J., Hill, A., Axelson, M., Moss, R. A., Goldberg, M. V., Cao, Z. A., Ledeine, J. M., Maglinte, G. A., Kopetz, S., & André, T. (2017). Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. The Lancet Oncology, 18(9), 1182–1191.
Paliwal, S., Kanagaraj, R., Sturzenegger, A., Burdova, K., & Janscak, P. (2014). Human RECQ5 helicase promotes repair of DNA double-strand breaks by synthesis-dependent strand annealing. Nucleic Acids Research, 42(4), 2380–2390.
Parkes, E. E., Walker, S. M., Taggart, L. E., McCabe, N., Knight, L. A., Wilkinson, R., McCloskey, K. D., Buckley, N. E., Savage, K. I., Salto-Tellez, M., McQuaid, S., Harte, M. T., Mullan, P. B., Harkin, D. P., & Kennedy, R. D. (2017). Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. Journal of the National Cancer Institute, 109(1), 199.
Parvathareddy, S. K., Siraj, A. K., Ahmed, S. O., Ghazwani, L. O., Aldughaither, S. M., Al-Dayel, F., Tulbah, A., Ajarim, D., & Al-Kuraya, K. S. (2021). PD-L1 protein expression in Middle Eastern breast cancer predicts favorable outcome in triple-negative breast cancer. Cells, 10(2), 229.
Patterson-Fortin, J., & D’Andrea, A. D. (2020). Exploiting the microhomology-mediated end-joining pathway in cancer therapy. Cancer Research, 80(21), 4593–4600.
Pelzer, N., Hoogeveen, E. S., Haan, J., Bunnik, R., Poot, C. C., van Zwet, E. W., Inderson, A., Fogteloo, A. J., Reinders, M. E. J., Middelkoop, H. A. M., Kruit, M. C., van den Maagdenberg, A., Ferrari, M. D., & Terwindt, G. M. (2019). Systemic features of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: A monogenic small vessel disease. Journal of Internal Medicine, 285(3), 317–332.
Permata, T. B. M., Hagiwara, Y., Sato, H., Yasuhara, T., Oike, T., Gondhowiardjo, S., Held, K. D., Nakano, T., & Shibata, A. (2019). Base excision repair regulates PD-L1 expression in cancer cells. Oncogene, 38(23), 4452–4466.
Pettitt, S. J., Frankum, J. R., Punta, M., Lise, S., Alexander, J., Chen, Y., Yap, T. A., Haider, S., Tutt, A. N. J., & Lord, C. J. (2020). Clinical BRCA1/2 reversion analysis identifies hotspot mutations and predicted neoantigens associated with therapy resistance. Cancer Discovery, 10(10), 1475–1488.
Pilger, D., Seymour, L. W., & Jackson, S. P. (2021). Interfaces between cellular responses to DNA damage and cancer immunotherapy. Genes & Development, 35(9–10), 602–618.
Pluciennik, A., Dzantiev, L., Iyer, R. R., Constantin, N., Kadyrov, F. A., & Modrich, P. (2010). PCNA function in the activation and strand direction of MutLα endonuclease in mismatch repair. Proceedings of the National Academy of Sciences, 107(37), 16066–16071.
Raats, D. A., Frenkel, N., van Schelven, S. J., Rinkes, I. H., Laoukili, J., & Kranenburg, O. (2017). CD95 ligand induces senescence in mismatch repair-deficient human colon cancer via chronic caspase-mediated induction of DNA damage. Cell Death & Disease, 8(3), e2669.
Reisländer, T., Lombardi, E. P., Groelly, F. J., Miar, A., Porru, M., Di Vito, S., Wright, B., Lockstone, H., Biroccio, A., Harris, A., Londoño-Vallejo, A., & Tarsounas, M. (2019). BRCA2 abrogation triggers innate immune responses potentiated by treatment with PARP inhibitors. Nature Communications, 10(1), 3143.
Reiss, K. A., Mick, R., Teitelbaum, U., O’Hara, M., Schneider, C., Massa, R., Karasic, T., Tondon, R., Onyiah, C., Gosselin, M. K., Donze, A., Domchek, S. M., & Vonderheide, R. H. (2022). Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. The Lancet Oncology, 23(8), 1009–1020.
Reyes, G. X., Kolodziejczak, A., Devakumar, L., Kubota, T., Kolodner, R. D., Putnam, C. D., & Hombauer, H. (2021). Ligation of newly replicated DNA controls the timing of DNA mismatch repair. Current Biology, 31(6), 1268-1276.e6.
Ribezzo, F., Shiloh, Y., & Schumacher, B. (2016). Systemic DNA damage responses in aging and diseases. Seminars in Cancer Biology, 37–38, 26–35.
Richard, I. A., Burgess, J. T., O’Byrne, K. J., & Bolderson, E. (2021). Beyond PARP1: the potential of other members of the poly (ADP-Ribose) polymerase family in DNA repair and cancer therapeutics. Frontiers in Cell and Developmental Biology, 9, 801200.
Riffell, J. L., Lord, C. J., & Ashworth, A. (2012). Tankyrase-targeted therapeutics: Expanding opportunities in the PARP family. Nature Reviews Drug Discovery, 11(12), 923–936.
Roh, J. S., & Sohn, D. H. (2018). Damage-associated molecular patterns in inflammatory diseases. Immune Network, 18(4), e27.
Roos, W. P., Thomas, A. D., & Kaina, B. (2016). DNA damage and the balance between survival and death in cancer biology. Nature Reviews Cancer, 16(1), 20–33.
Rospo, G., Lorenzato, A., Amirouchene-Angelozzi, N., Magrì, A., Cancelliere, C., Corti, G., Negrino, C., Amodio, V., Montone, M., Bartolini, A., Barault, L., Novara, L., Isella, C., Medico, E., Bertotti, A., Trusolino, L., Germano, G., Di Nicolantonio, F., & Bardelli, A. (2019). Evolving neoantigen profiles in colorectal cancers with DNA repair defects. Genome Med, 11(1), 42.
Sak, S. C., Harnden, P., Johnston, C. F., Paul, A. B., & Kiltie, A. E. (2005). APE1 and XRCC1 protein expression levels predict cancer-specific survival following radical radiotherapy in bladder cancer. Clinical Cancer Research, 11(17), 6205–6211.
Sallmyr, A., Tomkinson, A. E., & Rassool, F. V. (2008). Up-regulation of WRN and DNA ligase IIIalpha in chronic myeloid leukemia: Consequences for the repair of DNA double-strand breaks. Blood, 112(4), 1413–1423.
Sato, H., Niimi, A., Yasuhara, T., Permata, T. B. M., Hagiwara, Y., Isono, M., Nuryadi, E., Sekine, R., Oike, T., Kakoti, S., Yoshimoto, Y., Held, K. D., Suzuki, Y., Kono, K., Miyagawa, K., Nakano, T., & Shibata, A. (2017). DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nature Communications, 8(1), 1751.
Scaffidi, P., Misteli, T., & Bianchi, M. E. (2002). Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature, 418(6894), 191–195.
Scarpa, M., Ruffolo, C., Canal, F., Scarpa, M., Basato, S., Erroi, F., Fiorot, A., Dall’Agnese, L., Pozza, A., Porzionato, A., Castagliuolo, I., Dei Tos, A. P., Bassi, N., & Castoro, C. (2015). Mismatch repair gene defects in sporadic colorectal cancer enhance immune surveillance. Oncotarget, 6(41), 43472–43482.
Sen, T., Rodriguez, B. L., Chen, L., Corte, C. M. D., Morikawa, N., Fujimoto, J., Cristea, S., Nguyen, T., Diao, L., Li, L., Fan, Y., Yang, Y., Wang, J., Glisson, B. S., Wistuba, I. I., Sage, J., Heymach, J. V., Gibbons, D. L., & Byers, L. A. (2019). Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discovery, 9(5), 646–661.
Shen, J., Ju, Z., Zhao, W., Wang, L., Peng, Y., Ge, Z., Nagel, Z. D., Zou, J., Wang, C., Kapoor, P., Ma, X., Ma, D., Liang, J., Song, S., Liu, J., Samson, L. D., Ajani, J. A., Li, G. M., & LiangPeng, H. G. (2018). ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nature Medicine, 24(5), 556–562.
Shen, J., Zhao, W., Ju, Z., Wang, L., Peng, Y., Labrie, M., Yap, T. A., Mills, G. B., & Peng, G. (2019). PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Research, 79(2), 311–319.
Sheng, H., Huang, Y., Xiao, Y., Zhu, Z., Shen, M., Zhou, P., Guo, Z., Wang, J., Wang, H., Dai, W., Zhang, W., Sun, J., & Cao, C. (2020). ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. Journal for ImmunoTherapy of Cancer, 8(1), e000340.
Shukla, V., Halabelian, L., Balagere, S., Samaniego-Castruita, D., Feldman, D. E., Arrowsmith, C. H., Rao, A., & Aravind, L. (2020). HMCES functions in the alternative end-joining pathway of the DNA DSB repair during class switch recombination in B cells. Molecular Cell, 77(2), 384-394.e4.
Slade, D. (2020). PARP and PARG inhibitors in cancer treatment. Genes & Development, 34(5–6), 360–394.
Sloan, E. A., Ring, K. L., Willis, B. C., Modesitt, S. C., & Mills, A. M. (2017). PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including lynch syndrome-associated and MLH1 promoter hypermethylated tumors. American Journal of Surgical Pathology, 41(3), 326–333.
Spegg, V., Panagopoulos, A., Stout, M., Krishnan, A., Reginato, G., Imhof, R., Roschitzki, B., Cejka, P., & Altmeyer, M. (2023). Phase separation properties of RPA combine high-affinity ssDNA binding with dynamic condensate functions at telomeres. Nature Structural & Molecular Biology. https://doi.org/10.1038/s41594-023-00932-w.
Sugasawa, K., Okuda, Y., Saijo, M., Nishi, R., Matsuda, N., Chu, G., Mori, T., Iwai, S., Tanaka, K., Tanaka, K., & Hanaoka, F. (2005). UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell, 121(3), 387–400.
Sun, Y., Wang, J., Ma, Y., Li, J., Sun, X., Zhao, X., Shi, X., Hu, Y., Qu, F., & Zhang, X. (2021). Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. Journal of Experimental & Clinical Cancer Research, 40(1), 306.
Tang, Z., Pilié, P. G., Geng, C., Manyam, G. C., Yang, G., Park, S., Wang, D., Peng, S., Wu, C., Peng, G., Yap, T. A., Corn, P. G., Broom, B. M., & Thompson, T. C. (2021). ATR inhibition induces CDK1-SPOP signaling and enhances Anti-PD-L1 cytotoxicity in prostate cancer. Clinical Cancer Research, 27(17), 4898–4909.
Thorsson, V., Gibbs, D. L., Brown, S. D., Wolf, D., Bortone, D. S., Ou Yang, T. H., Porta-Pardo, E., Gao, G. F., Plaisier, C. L., Eddy, J. A., Ziv, E., Culhane, A. C., Paull, E. O., Sivakumar, I. K. A., Gentles, A. J., Malhotra, R., Farshidfar, F., Colaprico, A., Parker, J., & Shmulevich, S. I. (2018). The immune landscape of cancer. Immunity, 48(4), 812-830.e14.
Tiwari, V., Baptiste, B. A., Okur, M. N., & Bohr, V. A. (2021). Current and emerging roles of Cockayne syndrome group B (CSB) protein. Nucleic Acids Research, 49(5), 2418–2434.
Tobin, L. A., Robert, C., Nagaria, P., Chumsri, S., Twaddell, W., Ioffe, O. B., Greco, G. E., Brodie, A. H., Tomkinson, A. E., & Rassool, F. V. (2012). Targeting abnormal DNA repair in therapy-resistant breast cancers. Molecular Cancer Research, 10(1), 96–107.
Tokunaga, R., Xiu, J., Goldberg, R. M., Philip, P. A., Seeber, A., Battaglin, F., Arai, H., Lo, J. H., Naseem, M., Puccini, A., Berger, M. D., Soni, S., Zhang, W., Chen, S., Hwang, J. J., Shields, A. F., Marshall, J. L., Baba, H., Korn, W. M., & Lenz, H. J. (2020). The impact of ARID1A mutation on molecular characteristics in colorectal cancer. European Journal of Cancer, 140, 119–129.
Torres-Ramos, C. A., Johnson, R. E., Prakash, L., & Prakash, S. (2000). Evidence for the involvement of nucleotide excision repair in the removal of a basic sites in yeast. Molecular and Cellular Biology, 20(10), 3522–3528.
Tubbs, A., & Nussenzweig, A. (2017). Endogenous DNA damage as a source of genomic instability in cancer. Cell, 168(4), 644–656.
Tufegdžić Vidaković, A., Mitter, R., Kelly, G. P., Neumann, M., Harreman, M., Rodríguez-Martínez, M., Herlihy, A., Weems, J. C., Boeing, S., Encheva, V., Gaul, L., Milligan, L., Tollervey, D., Conaway, R. C., Conaway, J. W., Snijders, A. P., Stewart, A., & Svejstrup, J. Q. (2020). Regulation of the RNAPII pool is integral to the DNA damage response. Cell, 180(6), 1245-1261.e21.
Turajlic, S., Litchfield, K., Xu, H., Rosenthal, R., McGranahan, N., Reading, J. L., Wong, Y. N. S., Rowan, A., Kanu, N., Al Bakir, M., Chambers, T., Salgado, R., Savas, P., Loi, S., Birkbak, N. J., Sansregret, L., Gore, M., Larkin, J., Quezada, S. A., & Swanton, C. (2017). Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet Oncology, 18(8), 1009–1021.
Upadhyay, R., Boiarsky, J. A., Pantsulaia, G., Svensson-Arvelund, J., Lin, M. J., Wroblewska, A., Bhalla, S., Scholler, N., Bot, A., Rossi, J. M., Sadek, N., Parekh, S., Lagana, A., Baccarini, A., Merad, M., Brown, B. D., & Brody, J. D. (2021). A critical role for Fas-mediated off-target tumor killing in T-cell immunotherapy. Cancer Discovery, 11(3), 599–613.
Vélez-Cruz, R., Zadorin, A. S., Coin, F., & Egly, J. M. (2013). Sirt1 suppresses RNA synthesis after UV irradiation in combined xeroderma pigmentosum group D/Cockayne syndrome (XP-D/CS) cells. Proc Natl Acad Sci U S A, 110(3), E212–E220.
Wang, L., Mosel, A. J., Oakley, G. G., & Peng, A. (2012). Deficient DNA damage signaling leads to chemoresistance to cisplatin in oral cancer. Molecular Cancer Therapeutics, 11(11), 2401–2409.
Wang, L., Yang, L., Wang, C., Zhao, W., Ju, Z., Zhang, W., Shen, J., Peng, Y., An, C., Luu, Y. T., Song, S., Yap, T. A., Ajani, J. A., Mills, G. B., Shen, X., & Peng, G. (2020a). Inhibition of the ATM/Chk2 axis promotes cGAS/STING signaling in ARID1A-deficient tumors. The Journal of Clinical Investigation, 130(11), 5951–5966.
Wang, Z., Zhao, J., Wang, G., Zhang, F., Zhang, Z., Zhang, F., Zhang, Y., Dong, H., Zhao, X., Duan, J., Bai, H., Tian, Y., Wan, R., Han, M., Cao, Y., Xiong, L., Liu, L., Wang, S., & CaiWang, S. J. (2018). Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Research, 78(22), 6486–6496.
Wardlaw, C. P., & Petrini, J. H. J. (2022). ISG15 conjugation to proteins on nascent DNA mitigates DNA replication stress. Nature Communications, 13(1), 5971.
Waterman, D. P., Haber, J. E., & Smolka, M. B. (2020). Checkpoint responses to DNA double-strand breaks. Annual Review of Biochemistry, 89, 103–133.
Weitering, T. J., Takada, S., Weemaes, C. M. R., van Schouwenburg, P. A., & van der Burg, M. (2021). ATM: translating the DNA damage response to adaptive immunity. Trends in Immunology, 42(4), 350–365.
Wooster, R., Bignell, G., Lancaster, J., Swift, S., Seal, S., Mangion, J., Collins, N., Gregory, S., Gumbs, C., & Micklem, G. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature, 378(6559), 789–792.
Wright, W. D., & Heyer, W. D. (2014). Rad54 functions as a heteroduplex DNA pump modulated by its DNA substrates and Rad51 during D loop formation. Molecular Cell, 53(3), 420–432.
Wright, W. D., Shah, S. S., & Heyer, W. D. (2018). Homologous recombination and the repair of DNA double-strand breaks. Journal of Biological Chemistry, 293(27), 10524–10535.
Xian, S., Dosset, M., Almanza, G., Searles, S., Sahani, P., Waller, T. C., Jepsen, K., Carter, H., & Zanetti, M. (2021). The unfolded protein response links tumor aneuploidy to local immune dysregulation. EMBO Reports, 22(12), e52509.
Xiao, N., Wei, J., Xu, S., Du, H., Huang, M., Zhang, S., Ye, W., Sun, L., & Chen, Q. (2019). cGAS activation causes lupus-like autoimmune disorders in a TREX1 mutant mouse model. Journal of Autoimmunity, 100, 84–94.
Xie, A., Kwok, A., & Scully, R. (2009). Role of mammalian Mre11 in classical and alternative nonhomologous end joining. Nature Structural & Molecular Biology, 16(8), 814–818.
Xu, J., Lahiri, I., Wang, W., Wier, A., Cianfrocco, M. A., Chong, J., Hare, A. A., Dervan, P. B., DiMaio, F., Leschziner, A. E., & Wang, D. (2017). Structural basis for the initiation of eukaryotic transcription-coupled DNA repair. Nature, 551(7682), 653–657.
Yamazaki, T., Hannani, D., Poirier-Colame, V., Ladoire, S., Locher, C., Sistigu, A., Prada, N., Adjemian, S., Catani, J. P., Freudenberg, M., Galanos, C., André, F., Kroemer, G., & Zitvogel, L. (2014). Defective immunogenic cell death of HMGB1-deficient tumors: Compensatory therapy with TLR4 agonists. Cell Death and Differentiation, 21(1), 69–78.
Yang, X. W., Han, X. P., Han, C., London, J., Fishel, R., & Liu, J. (2022). MutS functions as a clamp loader by positioning MutL on the DNA during mismatch repair. Nature Communications, 13(1), 5808.
Yen, Y. T., Chien, M., Wu, P. Y., Ho, C. C., Ho, C. T., Huang, K. C., Chiang, S. F., Chao, K. S. C., Chen, W. T., & Hung, S. C. (2021). Protein phosphatase 2A inactivation induces microsatellite instability, neoantigen production and immune response. Nature Communications, 12(1), 7297.
Yilmaz, D., Furst, A., Meaburn, K., Lezaja, A., Wen, Y., Altmeyer, M., Reina-San-Martin, B., & Soutoglou, E. (2021). Activation of homologous recombination in G1 preserves centromeric integrity. Nature, 600(7890), 748–753.
Yousefzadeh, M. J., Wyatt, D. W., Takata, K., Mu, Y., Hensley, S. C., Tomida, J., Bylund, G. O., Doublié, S., Johansson, E., Ramsden, D. A., McBride, K. M., & Wood, R. D. (2014). Mechanism of suppression of chromosomal instability by DNA polymerase POLQ. PLoS Genetics, 10(10), e1004654.
Zhang, Q., Green, M. D., Lang, X., Lazarus, J., Parsels, J. D., Wei, S., Parsels, L. A., Shi, J., Ramnath, N., Wahl, D. R., Pasca di Magliano, M., Frankel, T. L., Kryczek, I., Lei, Y. L., Lawrence, T. S., Zou, W., & Morgan, M. A. (2019). Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Research, 79(15), 3940–3951.
Zhou, K. I., Peterson, B., Serritella, A., Thomas, J., Reizine, N., Moya, S., Tan, C., Wang, Y., & Catenacci, D. V. T. (2020). Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clinical Cancer Research, 26(24), 6453–6463.
Zhou, W., Richmond-Buccola, D., Wang, Q., & Kranzusch, P. J. (2022). Structural basis of human TREX1 DNA degradation and autoimmune disease. Nature Communications, 13(1), 4277.
Zighelboim, I., Goodfellow, P. J., Gao, F., Gibb, R. K., Powell, M. A., Rader, J. S., & Mutch, D. G. (2007). Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. Journal of Clinical Oncology, 25(15), 2042–2048.
Zimmer, A. S., Nichols, E., Cimino-Mathews, A., Peer, C., Cao, L., Lee, M. J., Kohn, E. C., Annunziata, C. M., Lipkowitz, S., Trepel, J. B., Sharma, R., Mikkilineni, L., Gatti-Mays, M., Figg, W. D., Houston, N. D., & Lee, J. M. (2019). A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. Journal for Immunotherapy of Cancer, 7(1), 197.
Acknowledgements
The work was funded by the National Key R & D Program of China (2022YFA1105200), Interdisciplinary Research Foundation of HIT, National Nature Science Foundation (Nos. 82025027, 82150115, 31871389 and 32000517), and Nature Science Foundation of Heilongjiang Province (No. YQ2021C024).
Author information
Authors and Affiliations
School of Life Science and Technology, Harbin Institute of Technology, Harbin, 150001, Heilongjiang, China
Zhiyuan Xiang, Hao Liu & Ying Hu
Corresponding author
Correspondence to Ying Hu.
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, Z., Liu, H. & Hu, Y. DNA damage repair and cancer immunotherapy. GENOME INSTAB. DIS. (2023). https://doi.org/10.1007/s42764-023-00098-1
Received21 December 2022
Revised05 February 2023
Accepted19 February 2023
Published31 March 2023
DOIhttps://doi.org/10.1007/s42764-023-00098-1
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkKeywords
DNA damage
DNA damage repair
Immunity
Cancer immunotherapy








用户登录
还没有账号?
立即注册