Common hotspots of cancer chemotherapy
Review Article
Adekunle Fiyin Ademikanra, Olutayo Micheal Oyewole & Azeemat Olanrewaju Olayiwola
Genome Instability & Disease 4, 181–196 (2023)
Abstract
Cancer is fundamentally a disturbance in the regulation of tissue development. Normal cells must undergo alterations in the genes responsible for cell proliferation and differentiation for cancer to arise. Carcinogenesis is the process through which cancer originates in healthy cells. It is characterized by cellular, genetic, and epigenetic alterations, as well as abnormal cell division. Oncogenes may be either normal genes with aberrant expression or mutant genes with surprising new abilities. Cancers are categorized with respect to the cell type involved in the cancerous growth. The categories include Carcinoma (Epithelial cells: lungs, breast, prostate, pancreas, intestine), Sarcoma (connective tissues: bone, cartilage, fat, nerves), Lymphoma & Leukaemia (immune system: lymph node & blood), Blastoma (embryonic cells), Germ cell tumour (ovarian & testicular germ cell). Cancer diagnosis involve an array of medical examinations such as blood count and X-ray imaging using electron endoscopy and computer-generated tomography. Tissue diagnosis reflects the histological grade, genetic abnormalities, and other properties of the proliferating cells which aid physicians in selecting the optimal course of treatment and determining the patient's prognosis. Cancer exhibits no visible symptoms except general symptoms such as unintended weight loss and fatigue. Chemotherapy refers to the use of one or more cytotoxic antineoplastic drugs (chemotherapeutic agents) in a specified treatment regime. It targets rapidly dividing cells, and its effectiveness is dependent on the type and severity of the disease. Classes of chemotherapeutic drugs are Alkylating agents, antimetabolites, Antitumor antibiotics, hormonal agents, plants alkaloids and miscellaneous agents. Other treatment of cancer includes the use of radiation, laser therapy, immunotherapy, and surgery. Cancer hotspots are areas of tumour DNA that are more susceptible to alteration. Examples of oncogenes with cancer hotspots include BRCA genes, p53 genes, APC genes, HER genes, PALB2 genes, ATM genes, ALK genes etc.
Introduction
Cancer's first description
Several papyri from Ancient Egypt include the first known descriptions of the condition we now refer to as cancer. In the Edwin Smith Papyrus, which dates to around 1600 BC, cancer, and a cauterization procedure for eliminating breast tumours are recorded (Topi et al., 2020). Hippocrates (ca. 460 BC–370 BC) called cancer Karakinos (carcinos) based on the appearance of the sliced surface of a solid malignant tumour with "the veins spread on all sides like the animal the crab has its foot," from which the disease receives its name. Cancer is derived from the Greek word karkinos, which Celsus (about 50 BCE to 50 CE) translated. While Hippocrates reserved carcinos for malignant tumours, the Greek physician Galen used oncos (the Greek word for swelling) to describe all tumours in the second century AD, Oncology may be traced to Galen, who coined the word (Thakur et al., 2021).
Earlier cancer diagnosis
Percivall Pott noticed in 1775 that chimney sweeps were more susceptible to getting scrotal cancer. Campbell De Morgan believed between 1871 and 1874, based on an 18th-century study, that the "cancer poison" spreads from the main tumour to distant areas through the lymph nodes. The use of surgery to cure cancer was ineffective owing to sanitary issues. In the nineteenth century, asepsis enhanced surgical cleanliness, and as survival rates increased, surgical excision of the tumour became the most common cancer therapy (Semalatha, 2018). Theodore Boveri discovered the genetic basis of cancer in 1902, he maintained that chromosomes were distinct and transmitted diverse heredity elements and that mutations of chromosomes may result in the formation of a cell with an endless growth potential that could be passed on. He theorized that radiation, physical or chemical assaults, and pathogenic bacteria might all have a role in developing or encouraging cancer (Burgio & Migliore, 2015).
Carcinogenesis
Carcinogenesis is the process through which cancer originates in healthy cells. This normal process which happens in almost all tissues and under a variety of conditions, is characterized by cellular, genetic, and epigenetic alterations, as well as abnormal cell division. A correct balance between cell division and cell death is necessary for the integrity of tissues and organs. In addition, environmental factors such as chemicals and radiation may cause mutations that may contribute to the development of cancer (Danforth, 2016).
Cancer is fundamentally a disturbance in the regulation of tissue development. Normal cells must undergo alterations in the genes responsible for cell proliferation and differentiation for cancer to arise. Genetic and epigenetic modifications include the gain or loss of a whole chromosome, a mutation affecting a single DNA nucleotide, and the silencing or activation of a microRNA that influences the expression of 100 to 500 genes. These alterations affect two key types of genes. Oncogenes may be either normal genes with aberrant expression or mutant genes with surprising new abilities. These cancer cells are more likely to develop malignant traits if they express these genes. Tumour suppressor genes function to inhibit the development, survival, and other properties of cancer cells (Kenneth, 2017; Maguire et al., 2015).
Classification of cancers
Cancers are classified according to the underlying cell type that is believed to have led to the tumour's growth (Ohashi et al., 2015);
Carcinomas are malignancies that arise from epithelial cells. Here, the majority of breast, prostate, lung, pancreatic, and colon cancers are categorized (Kim & Ryu, 2017).
Sarcoma: Cancers of connective tissue (including bone, cartilage, fat, and nerve) arise from mesenchymal cells that do not originate from the bone marrow (Uljanova & Ogneva, 2021).
Lymphoma and leukaemia are malignancies of the immune system, namely the lymph nodes and blood, which begin from blood-forming cells that move from the bone marrow (Lanka & Pulicherla, 2018).
Germ cell tumour Cancers of testicular or ovarian germ cells emerge from pluripotent cells (Maoz et al., 2020).
Blastomas are cancers that arise in underdeveloped embryonic tissue (Maoz et al., 2020).
Symptoms of cancer
Although cancer may cause more general symptoms such as weight loss or fatigue, they are often caused by the influence on the region of the body where it is developing. More than a hundred separate malignancies have been found, each with a huge array of possible symptoms and causes. In its early stages, cancer exhibits no visible symptoms. When a tumor enlarges or develops ulcers, symptoms, and signs manifest. The findings are contingent on the kind and stage of the cancer being treated. There are not many identifiable symptoms. A substantial proportion of occurrences occur in individuals who also have other illnesses or conditions, therefore, it is unusual for cancer patients to have had therapy for a range of diseases that physicians first thought to be the cause of their symptoms(Peart, 2017; Xing et al., 2019).
Local symptoms
The mass or ulceration of the tumour may produce local symptoms. Oesophageal cancer, for example, may cause the oesophagus to constrict, making swallowing difficult or painful. However, lung cancer, the mass effects may choke the bronchus, causing coughing or pneumonia. In the latter stages of cancer, there may be localized discomfort, but the swelling itself is often painless. Certain cancers might result in fluid build-up inside the chest or abdomen (Stephens & Aigner, 2016).
Systemic symptoms
General symptoms impact the whole body but have nothing to do with the main tumour or its metastases. Possible symptoms include unintentional weight loss, elevated body temperature, excessive fatigue, and skin changes. Cancers of the liver or kidney, in addition to Hodgkin's disease and leukaemia, may cause persistent fever (Alfano et al., 2018; Stephens & Aigner, 2016).
Diagnosis
Many cancers are first diagnosed via the appearance of symptoms or through screening. Without the study of a tissue sample by a pathologist, none of these approaches can provide a definitive diagnosis. Suspects of cancer are subjected to a battery of medical examinations. These include tests such as a blood count and an X-ray. Imaging using electron endoscopy and computed tomography.
Tissue diagnosis reflects the histological grade, genetic abnormalities, and other properties of the proliferating cells. Together, these pieces of information aid physicians in selecting the optimal course of treatment and determining the patient's prognosis.
Among the various types of tissue testing are cytogenetics and immunohistochemistry. These tests are crucial for learning more about molecular alterations that have occurred. From this, the appropriate course of treatment as well as a likely prognosis may be inferred (Ginsburg et al., 2020; Maringe et al., 2020).
Treatment methods for cancer
Cancer patients have access to several treatment options. Among the most often used therapies include surgery, chemotherapy, radiation therapy, hormone therapy, targeted therapy, and palliative care. When choosing the optimal course of treatment, both the patient's general health and individual preferences are taken into consideration (Namazi et al., 2015).
Chemotherapy
Chemotherapy refers to the use of one or more cytotoxic antineoplastic drugs (chemotherapeutic agents) in a specified treatment regimen. Standard chemotherapy medications target rapidly dividing cancer cells. The effectiveness of chemotherapy treatment depends on the kind and degree of the disease. In recent times, the outcomes of surgery coupled with chemotherapy have been favourable. Chemotherapy may cure cancers such as certain kinds of leukaemia but it is ineffective in certain circumstances, such as in most non-melanoma skin cancers, and some brain tumours. Frequently, the limited efficiency of chemotherapy is attributable to its toxicity to other tissues. When chemotherapy fails to eliminate a disease, it may help relieve symptoms such as pain or reduce an inoperable tumour so that surgery may be undertaken later (Amjad et al., 2021; Glasgow & Chougule, 2015).
Radiation
Radiation therapy makes use of ionizing radiation to treat or reduce symptoms. It works by damaging the DNA of cancerous tissue, killing it. With the intention of protecting healthy tissue, shaped radiation beams are directed from several exposure angles to cross at the malignancy, delivering a much higher dose there than in the surrounding healthy tissue. Like how chemotherapy affects different individuals differently, radiation therapy has diverse effects on various cancer types. About fifty per cent of the time, radiation therapy is the preferred treatment. Radiation may either originate from inside the body (through brachytherapy) or from the outside. Most often, low-energy x-rays are used to treat skin cancers, whereas higher-energy x-rays are employed to treat interior tumours. Usually, radiation therapy is performed in combination with surgery and/or chemotherapy. It may be sufficient in the early stages of some tumours, including head and neck cancer. Studies indicate that it is effective in treating painful bone metastases in around 70% of patients(Balaji et al., 2016; Lutz et al., 2017).
Surgery
Many localized solid tumours are treated with surgery, which may also provide palliative and survival advantages. Due to the requirement for biopsies, this procedure is often a crucial component of the final diagnosis and staging of a tumour. During surgery for localized cancer, the tumour and, in certain cases, the lymph nodes that drain it are often removed. This may be sufficient to remove certain kinds of cancer entirely (Song et al., 2017; Xia et al., 2022).
Immunotherapy
Several immunotherapies have been adopted since 1997, all to enhance or aid the immune system in its fight against cancer. Methods include antibodies, checkpoint therapy, and adoptive cell transfer (Ventola, 2017).
Laser therapy
Due to the powerful light emitted by the laser, malignant tumours and precancerous growths may be reduced or eradicated with laser therapy. Cancers of the skin and organ linings are the most common candidates for laser treatment. It is also used to treat cervical, penile, vaginal, vulvar, and non-small cell lung cancers in their early stages, in addition to basal cell skin cancer. It is often combined with other treatments, including surgery, chemotherapy, and radiation therapy. Lasers are safer, more effective, and leave less physical and emotional impact than traditional surgical techniques (Karampelas & Sloan, 2018; Nour-Eldin et al., 2017).
Cancer chemotherapy
Chemotherapy refers to the use of anti-cancer drugs (chemotherapeutic agents) to treat cancer throughout a predefined course of treatment. When administered to treat cancer, chemotherapy often combines many drugs. When utilized for additional reasons, such as life extension or symptom relief, chemotherapy may be used as a single agent. One of the primary subspecialties of medical oncology, which focuses on the use of medicines to treat cancer, is chemotherapy. Chemotherapy, usually known as "chemo," is the use of intracellular toxins to inhibit mitosis or cell division. This idea excludes more specific medications that suppress extracellular signalling. Hormonal therapies are the product of research into suppressing the signals of classical endocrine hormones that promote development. In contrast, targeted therapy refers to the inhibition of growth signals, such as those associated with receptor tyrosine kinases. Chemotherapeutics are often cytotoxic because they impede cell division. However, the susceptibility of cancer cells to various medicines varies substantially. Chemotherapy may be seen as a technique to damage or stress cells, which may result in cell death if apoptosis is induced. Damage to quickly proliferating cells in the bone marrow, digestive system, and hair follicles is a key source of chemotherapy's adverse effects (Gesmundo et al., 2022; Peng et al., 2017; Pérez-Herrero & Fernández-Medarde, 2015).
Classes of chemotherapeutic drugs
Cell cycle phase-specific agents are those that only have an impact on cells when they are in a certain phase of the cell cycle, as opposed to cell cycle phase-nonspecific agents, which have no such preference, they are most effective against malignancies when a large proportion of cells proliferate fast throughout the cell cycle. If a cell cycles rapidly through its most sensitive phase, it will be less vulnerable to the drugs (Mohammed & Ahamed, 2022; Tung et al., 2022).
Exposure to cells at any stage of the cell cycle is likely to result in the efficacy of non-phase-specific medications. Gap phase (phase G0) are equally as sensitive to damaging influences as actively dividing cells. Therefore, non-phase-specific medicines are among the most effective therapies for slow-growing cancers. Conventional anticancer medications are also categorised according to their origin or mechanism of action. According to research (Priestman, 2008),the following are the most prevalent classifications:
Alkylating and alkylating-like agents: Classic alkylating agents inhibit DNA replication by crosslinking, strand breaking, and abnormal base pairing. Their toxic effects may be seen at all phases of the cell cycle, but they are particularly destructive to rapidly dividing cells. Alkylating medicines, which target cells in G0 and encourage dormant cells to begin dividing again, may be used to shrink tumours. These cells are now sensitive to medications that target the cell cycle. There is evidence that these medications are effective against lymphomas, Hodgkin's disease, breast cancer, and multiple myeloma. These include cyclophosphamide, ifosfamide, chlorambucil, busulfan, and melphalan(Kim et al., 2016; Lajous et al., 2019).
Antimetabolites: Antimetabolites prevent the production of DNA and RNA by imitating fake metabolites that bind to the DNA strand or by inhibiting critical enzymes. The great majority of medications are only effective during the S phase of the cell cycle because they target groupings of cells that are continually dividing and replicating, these medicines are most effective against cancers with a rapid growth rate. The digestive and circulatory systems are also significantly impacted by the toxin. A few examples are methotrexate, 5-fluorouracil, and cytosine arabinoside(Kaur et al., 2022; Kovalev et al., 2022). For example, Methotrexate affects cancer and rheumatoid arthritis in two unique ways. Methotrexate is effective against cancer because it is a competitive inhibitor of dihydrofolate reductase (DHFR), an enzyme involved in tetrahydrofolate synthesis. Methotrexate binds to DHFR with 1,000 times the affinity of folate. DHFR is an enzyme that transforms inactive dihydrofolate into functional tetrahydrofolate. Folic acid is necessary for DNA synthesis as it is required for the de novo synthesis of the nucleoside thymidine. Folate is necessary for the synthesis of purine and pyrimidine bases; hence, its absence will result in a decrease in this process. Methotrexate inhibits the synthesis of nucleic acids, ribose nucleotides, thymidylates, and proteins. MTX also inhibits the attachment of interleukin 1-beta to its cell surface receptor (Kovalev et al., 2022; Liu et al., 2022).
Antitumor antibiotics: also known as anthracyclines, are used to treat cancer because they inhibit the creation of RNA and DNA. The cell cycle is a broad target for most medications however most susceptible to poisoning are the haematopoietic, digestive, cardiovascular, and reproductive systems. Acute electrocardiogram (ECG) changes and arrhythmias may be indicators of cardiac toxicity. Those who have a pre-existing heart problem are at the highest risk. Examples of chemotherapy drugs include bleomycin, daunorubicin, and doxorubicin (Liu & Zhao, 2022). Bleomycin causes DNA Strand breaks by inhibiting thymidine incorporation into DNA. According to in vitro experiments, oxygen and metal ions are essential for bleomycin to break down DNA. Bleomycin possibly chelates metal ions to generate a pseudoenzyme that reacts with oxygen to generate DNA-damaging superoxide and hydroxide radicals. Furthermore, these compounds facilitate the oxidation of lipids and other biological components. Due to their complementary and cumulative effects on DNA, bleomycin and doxorubicin are often used combined to treat Hodgkin's lymphoma. Similar to how doxorubicin relaxes topoisomerase complexes by targeting the topoisomerase II enzyme, bleomycin accomplishes the same by targeting the topoisomerase I enzyme (Bolzán & Bianchi, 2018).
Plant Alkaloids: During metaphase, plant alkaloids bind to microtubule proteins, stopping mitosis from progressing. This subparagraph focuses mostly on the M phase of the cell cycle. Particularly susceptible to poisoning are organs and processes relying on healthy blood, the immune system, the neurological system, and the reproductive system. Receiving these drugs carries a risk of hypersensitivity reactions. This class of medications comprises the vinca alkaloids, the epipodophyllotoxins and the taxanes (Dhyani et al., 2022; Xu et al., 2022). There are two proposed modes of action for vinblastine and other medicines that disrupt microtubules, such as colcemid and nocodazole. They inhibit microtubule mobility at low concentrations and reduce polymer size at high quantities. Recent research indicates that they aid in the separation of microtubule minus ends from their organizing centers. In addition, dose–response studies reveal that an increase in cytotoxicity correlates substantially with an increase in microtubule dissociation from spindle poles (Ghouse, 2020).
Hormonal agents: For these medications, the cell cycle phase is unimportant. Hormonal treatment may be used to treat breast, thyroid, prostate, and uterine cancers, among others. For hormones or hormone antagonists to be effective, the tumor cells must contain hormone receptors (e.g., oestrogen receptors in breast tumors) (Davis & Howard, 2019).
Miscellaneous agents are cytotoxic medications that do not fit neatly into any of the other categories. Asparaginase and hydroxyurea are two examples of these medications. If DNA is treated with topoisomerase inhibitors, single strand break repair and realignment are hindered or inhibited entirely. The toxin has negative effects on the cardiovascular and digestive systems as well. Topotecan and irinotecan are two of them (Davis & Howard, 2019).
Hotspots for chemotherapy in oncology
Cancer hotspots are areas of tumor DNA that are more susceptible to alteration. Panels such as ion ampliseq, Genowiz, and cantogene were designed specifically for finding hotspots. Cancer hotspot panels may focus on 49 genes and 2800 of the most frequent mutations that have been widely studied or are generally acknowledged to include information important in the diagnosis, prognosis, or treatment of human oncogenes (Naito et al., 2018). Details about this genes and mutations can be found in the appendix section.
The role of mutations in genes in cancer
BRCA genes
In about 70–80% of instances, mutations in the BRCA genes result in protein malfunction or absence of protein product (Cassidy et al., 2014; Moynahan, 2002). These mutations were related by clinical evidence to an increased risk of inherited malignancies (Cal et al., 2010). There is broad consensus that mutations in the BRCA genes are significant factors to breast cancer. However, not all mutations result in faulty proteins; hence, they cannot be relied upon as a reliable prediction of cancer. As a result, much work has been devoted to calculating the cancer risk associated with the whole range of potential BRCA mutations. Due to the diversity of effects mutations may have on BRCA gene function and the lack of agreement as to whether or not they are cancer-causing, this has proven difficult for researchers (Miki et al., 1994).
After discovering BRCA1 in 1994, scientists quickly discovered BRCA2 in 1995. Following this point, regular clinical use of genetic testing for breast cancer risk began identifying a mutation prior to a cancer diagnosis might prevent the development of breast and ovarian cancers. Therefore, women with the BRCA mutation may be eligible for better breast cancer screening to detect the illness early and maybe prevent its spread to other organs. In addition, women with the BRCA mutations who develop breast (or ovarian) cancer may need a different treatment approach than those without these mutations. Women with a family history of breast cancer must have access to genetic testing at the time of diagnosis in order to get the tailored treatment they need to survive (Wooster et al., 1995) (Fig. 1, 2, 3, 4 and 5).
Fig. 1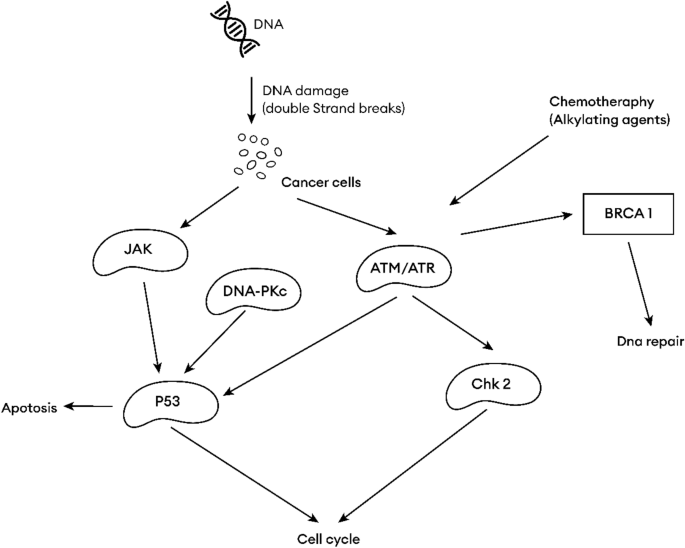
Graphical abstract of common hotspot in cancer chemotherapy
Fig. 2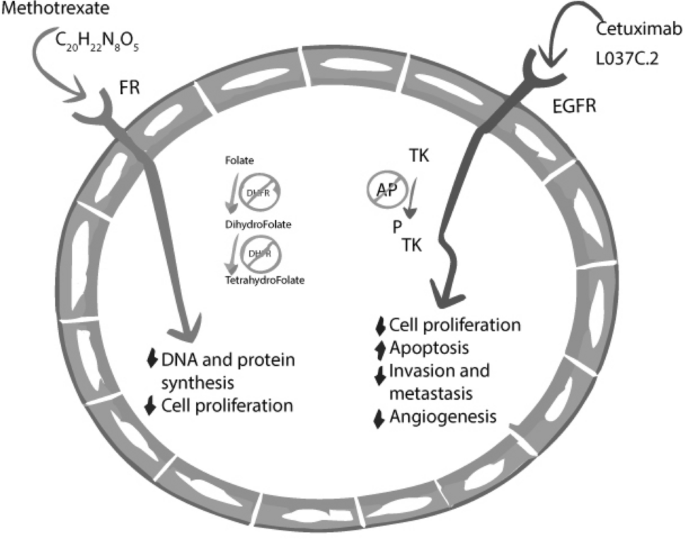
Mechanism of action of Methotrexate & Cetuximab (Kis et al., 2022)
Fig. 3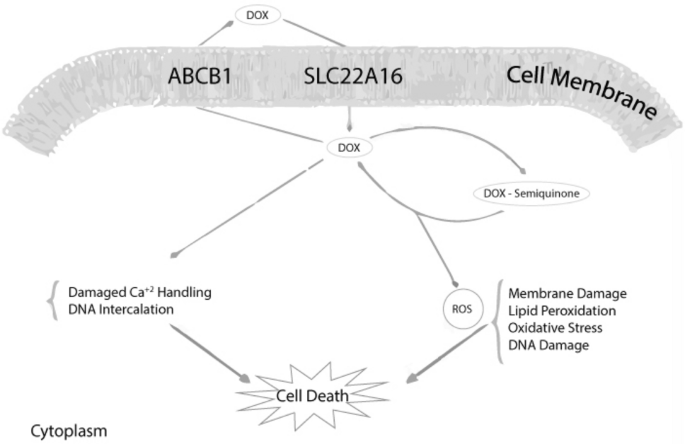
Mechanism of action of Doxorubincin (Faraji et al., 2016)
Fig. 4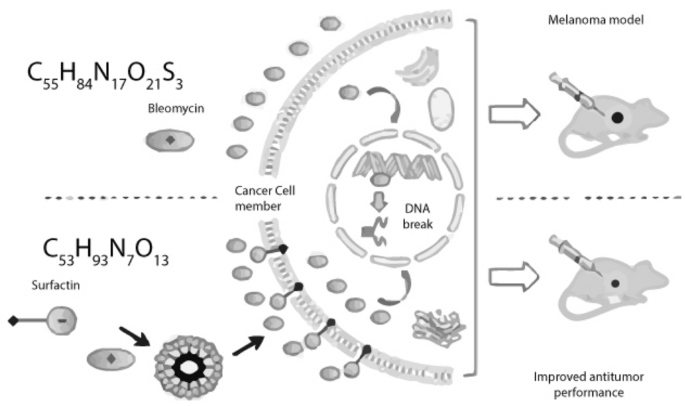
Mechanism of action of Bleomycin and the action of surfactin on its antitumor performance (Faraji et al., 2016)
Fig. 5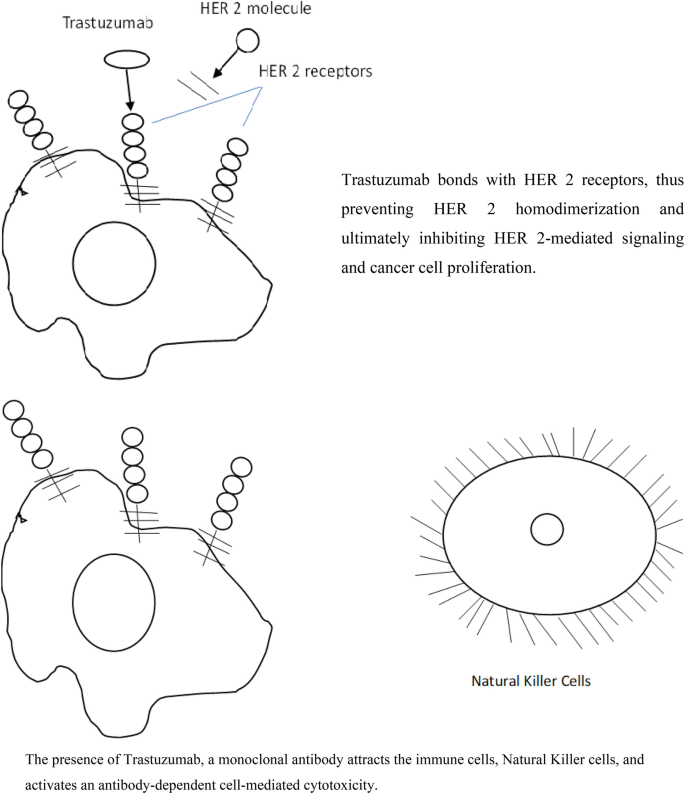
Mechanism of action of trastuzumab
Approximately 1600 mutations in the BRCA1 gene have been identified so far. These modifications may occur anywhere in the gene, including coding and noncoding regions (Godet & Gilkes, 2017). Some of these mutations are known as "founder mutations" because they tend to develop in relatively tiny, genetically unique groups. Most BRCA1 mutations occur in exons 11–13 of the gene, which code for BRCA1-important NLS and binding sites for many BRCA1-interacting proteins, including c-Myc, Rad50, pRb, Rad51, BRCA2, and PALB2 (Clark et al., 2012). Exons 11 and 13 mutations of the BRCA1 gene have been associated with an increased risk of breast and ovarian cancer. The great majority of BRCA1 gene mutations are caused by frameshift insertions/deletions, nonsynonymous truncations, and splicing site disruptions, resulting in either missense mutations or the creation of nonfunctional proteins (Karami & Mehdipour, 2013). Exons 11 and 13 Mutations in the BRCA1 gene increase the risk of breast, ovarian, and prostate cancers in both sexes. Carriers of the BRCA1 mutation are at higher risk for many forms of cancer, including colon, rectal, pancreatic, and stomach cancers (Brose et al., 2002; Thompson & Easton, 2004; Thompson et al., 2002).
Almost 1800 BRCA2 mutations have been found to far (Godet & Gilkes, 2017). The most prevalent forms of mutations that induce protein truncation or inactivation include insertions, deletions, and nonsense mutations (Fackenthal & Olopade, 2007). Ashkenazi Jews were discovered to have the greatest prevalence of the frameshift mutation 6174delT in the BRCA2 gene (Walsh et al., 2017).
p53 gene mutations
Since its mutations were discovered to be the unifying factor in more than half of all malignancies in 1979, the TP53 gene and protein have been in the limelight (Sallman et al., 2020). TP53 mutations provide a unique genetic dataset with a dismal survival rate in acute myeloid leukemia (AML). Approximately 5% of Li Fraumeni-related malignancies develop AML (Amadou et al., 2018) and those who inherit a single TP53 gene mutation are likely to acquire Li-Fraumeni syndrome. Mutations in the TP53 transcription factor are among the most common somatic changes seen in human malignancies. Only around 5–10% of newly diagnosed cases of AML include these mutations, but in around 25% of patients aged 60–67 (Olivier et al., 2006, 2010).
TP53 Variations and Breast Cancer Somatic TP53 missense mutations account for about half of all human cancers (Petitjean et al., 2007; Soussi, 2007). Despite the fact that the great majority of TP53 mutations are situated in the protein's DNA-binding domain, whole-genome sequencing has discovered mutations at the N and C termini of TP53 (Vousden & Lu, 2002). Mutations in TP53 may be present in 20% to 35% of breast tumors, with frequency varying by tumor type (Turpin et al., 2002). Approximately 1,400 breast cancers have been associated with TP53 mutations (Olivier et al., 2006, 2010) and due to their inability to transactivate, the negative effects of p53 mutations may be more widespread (Willis et al., 2004). Mutations in p53 may either have no influence on the protein's function or result in enhanced apoptotic activity relative to the gene's wild-type version (Friedlander et al., 1996; Rowan et al., 1996). If they are more stable and nuclear-localized, immunohistochemistry may be able to identify mutant p53 proteins in tumor cells. This is likely because the altered p53 protein cannot stimulate the synthesis of MDM2 protein, which is needed for its own planned death. This procedure may be more complicated than it seems at first sight. p53 mutations and other clinicopathological characteristics have been widely explored. Immunostaining for p53 is associated with the absence of estrogen and progesterone receptor expression in a breast tumor. They are associated with proliferation, histological grade, aneuploidy, and lower survival periods (Bonnefoi et al., 2003; Hensel et al., 2002; Malamou-Mitsi et al., 2006).
There are a number of diagnostic tests that can determine a patient's chemosensitivity quickly enough to allow for treatment planning before the disease progresses too far. The design of pharmacogenomic predictors is centered on the patient (Hess et al., 2006). In contrast, tumor-based testing employs in vitro response assays and molecular profiling (Kim & Ryu, 2017; Kim et al., 2016; Takamura et al., 2002). It is postulated that genotoxic stress, such as that generated by cytotoxic treatment, induces apoptosis through a p53-dependent mechanism (Herr & Debatin, 2001). No definitive answer has yet been found to the question of whether a patient's p53 status can be utilized as a predictor of their response to chemotherapy, despite more than 20 years of study. Due to the complexity of the influencing forces, it is doubtful that a simple explanation can be relied upon. Ex vivo investigations have shown consistent results independent of the treatment technique.
Mutations in zinc-binding domains predicted early recurrence and were linked to initial resistance to therapy in a trial of 63 adults with locally advanced breast tumors treated with neoadjuvant doxorubicin (Aas et al., 1996). The same researchers repeated the same findings in a bigger study including 90 patients (Geisler et al., 2003). Berns et al. found that patients with advanced breast cancer who had tamoxifen or first chemotherapy did poorly on anthracycline-based chemotherapy (Berns et al., 2007). Rahko et al. evaluated the predictive significance of TP53 mutations using 254 samples from patients with primary breast cancer (Rahko et al., 2003). Patients with advanced disease who tested positive for p53 had a poor response to anthracycline-based therapy.
Moreover, even though cancers with a TP53 mutation often have a worse prognosis, these tumors have been linked to greater overall survival rates. Rouzier et al. observed that cancers with a basal-like character were more sensitive to paclitaxel and doxorubicin (Rouzier et al., 2005). Paclitaxel and doxorubicin were shown to be especially effective against tumors having a basal nature, as revealed by expression profiling (Kröger et al., 2006).
Adenomatous Polyposis Coli (APC) gene
The absence of a viable adenomatous polyposis coli (APC) tumor suppressor gene has been linked to colorectal cancer, the second leading cause of cancer-related mortality after lung cancer. The APC mutation is related with cancers of the stomach, breast, lungs, and brain in addition to colon cancer (Fang et al., 2022). The chromosome 5q APC gene works as a negative regulator of the β-catenin/ WNT pathway. Active Wnt is a growth-stimulating protein that permits changes in cellular processes, such as the overexpression of β-catenin and transcription of Wnt target genes, both of which have been associated with the onset of invasive illnesses (Rubinfeld et al., 1993). Mutations in the APC gene result in the inhibition of the formation of a complex involving APC protein, Axin/Axin2, glycogen synthase kinase-3b (GSK-3b), and casein kinase 1, which is responsible for the degradation of β-catenin. This leads to the accumulation of β-catenin, which can promote the development of malignancies (Behrens et al., 1998; Sakanaka et al., 1998; Sparks et al., 1998).
In epithelial cells, chromosomal instability (CIN) occurs from a chain reaction. Loss of APC heterozygosity and CIN are both linked to changes in the DNA that are early signs of cancer in colorectal cancer. These changes are called hypomethylation and hypermethylation (Rao & Yamada, 2013). Reduced APC gene activity and β-catenin accumulation correlate with diminished E-cadherin expression and, by extension, intracellular adhesion (Smits et al., 2000). Mutations in the kras gene result in the formation of polyps or benign adenomas in the colon, while the deletion of the tumor suppressor genes p53 leads to cancer (Smith et al., 2013).
The APC gene may be affected by many things, such as genetics, diet, age, metabolic syndrome, and colon inflammation. Colitis-associated cancer (CAC) is rare when the p53 (APC) gene has been altered, but it is prominent in colorectal malignancies when APC is absent (Chatila et al., 2023). Possible causes include oxidative damage that triggers the release of inflammatory cytokines like IL-6, which then affect dysplastic and neoplastic growth (1.5–2.0 times the normal risk). Due to the association between increased cytokines and disease, individuals with obesity may need fewer somatic mutations to develop microsatellite-instable colon cancer. Overweight women seem to get colorectal cancer at an earlier age than other categories (Liu et al., 2022). Due to persistent inflammation, obese individuals may be more susceptible to the activation of signaling pathways (such as JAK/STAT) that govern cancer, this may be the case, but further investigation is required (Bordonaro & Lazarova, 2015; Stone et al., 2018).
Familial adenomatous polyposis (FAP) is an autosomal dominant malignancy that is transmitted from parent to kid, and research has connected it to the APC gene. If patients' illnesses are not handled, it is possible for them to develop cancers of the duodenum, pancreas, thyroid, liver, and central nervous system. Colorectal cancer is the most frequent lethal result of untreated disease (Tabernero et al., 2022). In teenagers, the loss of heterozygosity for the APC gene is linked to the fast growth of hundreds of premalignant polyps. Most individuals with APC deficiency have a germline mutation between codons 1061 and 1309, but another mutation renders both APC alleles ineffective (Galiatsatos & Foulkes, 2006). Patients in their forties and fifties often need preventative colectomies owing to the increased incidence of colorectal cancer at those ages (Galiatsatos & Foulkes, 2006; Tudyka & Clark, 2012). Although certain APC mutations may be inherited, the overwhelming majority arise spontaneously. Patients with FAP who have a germline mutation outside of this region may have a second tumor-causing mutation in the MCR gene (Mori et al., 1994; Rowan et al., 2000). By uncovering novel therapeutic targets, genomic sequencing has the potential to help in the treatment of hereditary and sporadic forms of colorectal cancer (Esplin & Snyder, 2014).
Mutations in the APC gene cause several types of FAP, such as attenuated FAP (AFAP), Gardner syndrome, and Turcot syndrome. AFAP is a subtype of FAP produced by mutations in the APC gene and characterized by a decreased risk of colorectal cancer, a delayed onset of symptoms, and fewer polyps. Polyp growth is thought to be the result of a third hit, or mutation, on a germline allele. Polyp development may be caused by a third hit or mutation on a germline allele, leading to a milder version of the disease (Spirio et al., 1998). Gardner syndrome is a form of FAP characterized by the presence of premalignant polyps in the colon and extracolonic features including desmoid tumors, osteomas, fibromas, epidermal cysts, and ocular involvement (Kim et al., 2020). Patients with Turcot syndrome, which is associated with both FAP and hereditary non-polyposis colorectal cancer (HNPCC), present with primary brain tumors such as glioblastoma or medulloblastoma (Paraf et al., 1997). Despite their various appearances, many illnesses have a common risk factor for colon cancer.
For individuals with APC-mutated colorectal cancer, a growing number of studies detail a variety of therapy options (Kim et al., 2020). The 3-year survival rate for patients in stages II and III of colorectal cancer treated with the conventional chemotherapy combination of leucovorin, 5-fluorouracil, and oxaliplatin (FOLFOX) was around 78.2% (Andre, 2004). Current therapies fall far short of the high mortality rates in the United States (approximately 50,000 deaths per year) (Siegel et al., 2019, 2021). Targeted treatment options are limited in tumors with APC inactivation, and survival benefits are at best minor.
The human epidermal growth factor receptor (HER)
Overexpression of the HER2 protein is associated with reduced overall survival and resistance to several chemotherapeutic treatments in 25% of breast tumors.
It is often believed that overexpression of the HER2 protein results from gene amplification. Multiple in vitro studies have shown that transfection of HER2 into mammary epithelial cells results in the transformation of those cells into malignant tumors, supporting the concept that overexpression of HER2 is a risk factor for the development of breast cancer (Pierce et al., 1991). In contrast, it has been shown that the HER2-HER3 heterodimer complex is required for HER2 to promote breast cancer cell growth (Holbro et al., 2003).
Trastuzumab primarily functions by inhibiting intracellular signaling and activating the immune system to target cancer cells. Given that HER2 is known to initiate several signaling pathways, it appears logical that its inhibition would have a comparable impact to its activation. Trastuzumab may lower HER2 activity in part by promoting the internalization and destruction of HER2 (Menard et al., 2003; Rubin & Yarden, 2001). Recent study indicates that Trastuzumab inhibits cell proliferation in HER2-overexpressing cells via affecting the PI3K-Akt signaling pathway (Junttila et al., 2009; Lee-Hoeflich et al., 2008).
Trastuzumab alone, in vitro, does not result in substantial apoptosis (Marches & Uhr, 2004; Yakes et al., 2002). Numerous in vivo investigations demonstrate that trastuzumab mediates the generation of immunological responses such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (Hynes & Lane, 2005). ADCC entails the detection and attachment of immune-effector cells expressing the Fcc receptor to the Fc domain of an IgG1 antibody (trastuzumab), followed by the destruction of the cells (in this case, tumor cells) linked to the antibody. When treating tumors with trastuzumab in mice lacking the FC receptor (FcR) and comparing the results to those seen in FC receptor-expressing animals, the relevance of ADCC to trastuzumab-induced anticancer efficacy is perhaps most convincingly shown (Clynes et al., 2000). However, it remains difficult to demonstrate conclusively in breast cancer patients that ADCC plays a role in trastuzumab's activity, as demonstrated by the finding by Gennari et al. (Gennari et al., 2004) that lymphoid cell infiltration was higher in tumor samples collected after trastuzumab treatment than in pretreatment tumor specimens.
PALB2
Gene PALB2 encodes the PALB2 (partner and localizer of BRCA2) protein. This protein facilitates the co-localization of BRCA1 and BRCA2 in DNA-damaged areas, where they may function as a cancer-fighting team (Zhang et al., 1996). The N-terminal coiled-coil domain of PALB2 facilitates interaction with BRCA1, whereas the C-terminal WD40 repeat domain mediates binding to BRCA2. A motif related to chromatin is situated in the middle of PALB2. The great majority of the more than 900 pathogenic/likely pathogenic changes identified so far in PALB2 are truncating variants (nonsense or frameshift), however missense variants and huge genomic rearrangements have also been seldom reported. Carriers with pathogenic PALB2 mutations have an enhanced risk of breast cancer, while ovarian and pancreatic cancer risk is only marginally raised (PALB2). Fanconi anemia subtype FA-N was revealed to contain biallelic mutations in the PALB2 gene, and it was later shown that pathogenic mutations in PALB2 were associated with hereditary breast cancer (Xia et al., 2007).
In Finland, a founder PALB2 c.1592delT mutation was detected in 3 of 113 (2.7%) familial breast cancer patients and 18 of 1,918 (0.9%) unselected breast cancer cases. This mutation is associated with a fourfold increased hereditary susceptibility for female breast cancer (Erkko et al., 2007). Recent study indicates that women with the PALB2 mutation had a 9.47-fold increased risk of developing breast cancer compared to the general population. A woman with a mutant PALB2 gene has a 14% chance of developing breast cancer by age 50 and a 35% chance by age 70. Having the PALB2 gene variant increases a woman's risk of developing breast cancer, however this risk varies with age and family history. Between the ages of 20 and 39, women with the PALB2 mutation had an 8–9 times higher risk of developing breast cancer than the general population, a 6–8 times higher risk between the ages of 40 and 60, and a 5 times higher risk beyond the age of 60. Women with a PALB2 mutation who are 70 years old and have no family history of the disease have a 33% probability of having breast cancer, whereas women with a significant family history of the disease among first-degree relatives have a 58% likelihood. According to studies (Antoniou et al., 2014), due to the fact that PALB2 acts in the same pathway where BRCA1 and BRCA2 are active in DNA-damage repair, mutations in PALB2 may have equivalent effects on different cancers as mutations in BRCA1 and BRCA2.. Therefore, it was proposed that individuals at risk for developing breast cancer who lacked BRCA1 or BRCA2 gene mutations, as well as male breast cancer patients, should undergo testing for PALB2 gene mutations (Southey et al., 2016).
ATM genes
The protein encoded by this gene is a ATM serine/threonine kinase and a member of the PI3/PI4-kinase family. It comprises a total of 67 exons and is located at location 11q22.3 in the human genome. This protein phosphorylates and controls a variety of other proteins, such as the tumor suppressors p53 and BRCA1, the cell cycle checkpoint kinase CHK2, the cell cycle checkpoint proteins RAD17 and RAD9, and the DNA repair protein NBS1. It is believed that this protein and the closely related kinase ATR are master regulators of cell cycle checkpoint signaling pathways that are necessary for cellular response to DNA damage and genomic integrity. This gene mutation is associated with the autosomal recessive disorder ataxia telangiectasia. Lymph nodes, the urine bladder, the appendix, the thyroid, the stomach, the skin, etc. express the proteins (Solyom, 2011).
The C-terminal phosphatidylinositol 3-kinase (PI3-kinase) domain of ATM was the first differentiating feature found, This domain links ATM to other proteins that govern cell cycle progression and detect DNA damage (Kim et al., 2022). In response to DNA damage, In response to DNA damage, these proteins likely phosphorylate one or more substrates (including p53, c-Abl, RPA, and PHAS-1) to activate radiation signal transduction pathways and/or recruit proteins to DNA repair sites (Ghosh & Ghosh, 2021). ATM participates in the activation of cell cycle checkpoints, DNA repair, and induction of apoptosis as physiological responses to DNA breaks. It is probably a sensor for a specific kind of strand break, such as those generated by free radicals or DNA recombination, and subsequently activates a number of critical regulators of the DNA damage response and initiates a number of signaling cascades in response to the damage (Ghosh & Ghosh, 2021). Women with a mutation in the ATM gene are believed to have a 20% to 60% increased risk of breast cancer (Wendt & Margolin, 2019). Individuals with the ATM gene mutation are more prone to develop breast cancer at a younger age or in both breasts. A mutation of the ATM gene has been linked to an increased risk of breast cancer, but it is unknown if this also raises the risk of other forms of cancer. Additionally, there may be an increased risk of radiation sensitivity, as shown by a number of studies (Sibilio et al., 2022).
ALK
Anaplastic lymphoma kinase is a receptor tyrosine kinase belonging to the insulin receptor superfamily is encoded by this gene. This gene plays a critical role in normal brain development by significantly impacting specific types of neurons in the nervous system. Although its expression is temporary in certain regions of the brain and spinal cord, it is still essential for the development of the nervous system (Souttou et al., 2019). As a negative regulator of white adipose tissue lipolysis and sympathetic tone, it controls energy expenditure and helps maintain optimum energy balance, and it plays an essential role in hypothalamic neurons' resistance to weight gain (Reshetnyak et al., 2018). Additionally, pleiotrophin (PTN) and midkine (MDK) may have a role in regulating ALK activation (Xu et al., 2014). PTN binding activates the mitogen-activated protein kinase (MAPK) pathway, which is essential for anti-apoptotic signaling and regulation of cell growth (Xu et al., 2014). IRS1 and AKT serine/threonine kinase activation likely contribute to NF-kappaB activation by ALK genes (Motegi et al., 2004). MDK autocrine signaling for growth and survival involves the recruitment of IRS1 to activated ALK, which then activates NF-kappaB (Motegi et al., 2004).
This gene has been associated to cancers including anaplastic large cell lymphomas, neuroblastoma (neuroblastoma 3 and neuroblastoma), and non-small cell lung cancer. The most prevalent genetic changes in this gene are chromosomal rearrangements, which result in the formation of numerous fusion genes implicated in tumorigenesis. ALK mutations have been shown to provide resistance to current tyrosine kinase inhibitors, notwithstanding crizontinib's efficacy. It has been demonstrated that the HSP90 inhibitor 17-AAG is cytostatic in ALK-mutated cell lines; nevertheless, the treatment of these resistant patients with second-generation TKIs has had a mixed effect (Shaw & Engelman, 2013).
Conclusion
Understanding genetics, pharmaceutical use, and the application of a chemotherapy strategy are critical for the treatment or management of cancer. Cancer is a term used to classify a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. Cancer is fundamentally a disturbance in the regulation of tissue development and the causes are not entirely understood. Although in the early stages, cancers do not exhibit visible symptoms, cancer symptoms may vary and largely depend on the type and the region of the body where it is developing. One of the most common treatment options for cancer patients is chemotherapy. Chemotherapy is the use of anti-cancer drugs to treat cancer over a specified treatment period. Cancer hotspots are areas of tumor DNA that are more susceptible to alteration. There are about 49 genes that have been implicated and widely accepted to contain information that may lead to more breakthroughs in the diagnosis and treatment of cancer. These genes include but are not limited to the following: BRCA genes, TP53, APC, HER, PALB2, ALK and the ATM gene.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
DNA:
Deoxyribonucleic Acid
BRCA:
Breast Cancer
p53:
Tumor protein 53
APC:
Adenomatous Polyposis Coli
HER:
Human Epidermal growth factor Receptor
PALB2:
Partner and Localizer of BRCA2
ATM:
Ataxia-telangiectasia Mutated.
ALK:
Anaplastic Lymphoma Kinase
BC:
Breast Cancer
RNA:
Ribonucleic Acid
G0:
G0 phase (in cell cycle)
DHFR:
Dihydrofolate Reductase
MTX:
Methotrexate
ECG:
Electrocardiogram
NLS:
Nuclear Localization Signal
c-Myc:
Myc Proto-Oncogene
RAD50:
DNA repair protein RAD50
PRB:
Retinoblastoma protein
RAD51:
DNA repair protein RAD51
AML:
Acute Myeloid Leukemia
MDM2:
Mouse Double Minute 2 Homolog
CK1:
Casein Kinase 1
GSK-3b:
Glycogen Synthase Kinase-3 Beta
CIN:
Chromosomal Instability
CAC:
Coiled-Coil Domain Containing
JAK:
Janus Kinase
STAT:
Signal Transducer and Activator of Transcription
FAP:
Familial Adenomatous Polyposis
AFAP:
Attenuated Familial Adenomatous Polyposis
HNPCC:
Hereditary Non-Polyposis Colorectal Cancer
PI3K-Akt:
Phosphatidylinositol-3-kinase-protein kinase B
ADCC:
Antibody-Dependent Cellular Cytotoxicity
IgG1:
Immunoglobulin G1
FA-N:
Focal Adhesion Network
PTN:
Pleiotrophin
MDK:
Midkine
MAPK:
Mitogen-Activated Protein Kinase
IRS1:
Insulin Receptor Substrate 1
NF-KappaB:
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
HSP90:
Heat Shock Protein 90
TKIs:
Tyrosine Kinase Inhibitors
References
Aas, T., Børresen, A. L., Geisler, S., Smith-Sørensen, B., Johnsen, H., Varhaug, J. E., et al. (1996). Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nature Medicine., 2(7), 811–814.
Alfano, C.M., Kevorkian, C.G., Miller, K.D., Siegel, R.L., Khan, R., & Jemal, A., et al. (2018). Cancer Rehabilitation [Internet]. Stubblefield MD, editor. Springer Publishing Company. 967 p. Available from: https://connect.springerpub.com/content/book/978-0-8261-2164-6
Amadou, A., Waddington Achatz, M. I., & Hainaut, P. (2018). Revisiting tumor patterns and penetrance in germline TP53 mutation carriers: Temporal phases of Li–Fraumeni syndrome. Current Opinion in Oncology., 30(1), 23–29.
Amjad, M.T., Chidharla, A., & Kasi, A. (2021). Cancer chemotherapy. In: StatPearls [Internet]. StatPearls Publishing.
Andre, T. (2004). Multicenter international study of oxaliplatin/5-fluorouracil/leukovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators: Oxaliplatin, fluorouracil and leukovorin as adjuvant treatment for colon cancer. New England Journal of Medicine, 50, 2243–2251.
Antoniou, A. C., Casadei, S., Heikkinen, T., Barrowdale, D., Pylkäs, K., Roberts, J., et al. (2014). Breast-cancer risk in families with mutations in PALB2. New England Journal of Medicine., 371(6), 497–506.
Balaji, K., Subramanian, B., Yadav, P., Radha, C. A., & Ramasubramanian, V. (2016). Radiation therapy for breast cancer: Literature review. Medical Dosimetry., 41(3), 253–257.
Behrens, J., Jerchow, B. A., Würtele, M., Grimm, J., Asbrand, C., Wirtz, R., et al. (1998). Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science, 280(5363), 596–599.
Berns, K., Horlings, H. M., Hennessy, B. T., Madiredjo, M., Hijmans, E. M., Beelen, K., et al. (2007). A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell, 12(4), 395–402.
Bolzán, A. D., & Bianchi, M. S. (2018). DNA and chromosome damage induced by bleomycin in mammalian cells: An update. Mutation Research/reviews in Mutation Research., 775, 51–62.
Bonnefoi, H., Diebold-Berger, S., Therasse, P., Hamilton, A., Van De Vijver, M., MacGrogan, G., et al. (2003). Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: Are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Annals of Oncology., 14(3), 406–413.
Bordonaro, M., & Lazarova, D. (2015). Hypothesis: Obesity is associated with a lower mutation threshold in Colon Cancer. Journal of Cancer., 6(9), 825.
Brose, M. S., Rebbeck, T. R., Calzone, K. A., Stopfer, J. E., Nathanson, K. L., & Weber, B. L. (2002). Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. Journal of the National Cancer Institute., 94(18), 1365–1372.
Burgio, E., & Migliore, L. (2015). Towards a systemic paradigm in carcinogenesis: Linking epigenetics and genetics. Molecular Biology Reports., 42(4), 777–790.
Cassidy, L. D., Liau, S. S., & Venkitaraman, A. R. (2014). Chromosome instability and carcinogenesis: Insights from murine models of human pancreatic cancer associated with BRCA2 inactivation. Molecular Oncology., 8(2), 161–168.
Chatila, W. K., Walch, H., Hechtman, J. F., Moyer, S. M., Sgambati, V., Faleck, D. M., et al. (2023). Integrated clinical and genomic analysis identifies driver events and molecular evolution of colitis-associated cancers. Nature Communications., 14(1), 110.
Clark, S. L., Rodriguez, A. M., Snyder, R. R., Hankins, G. D., & Boehning, D. (2012). Structure-function of the tumor suppressor BRCA1. Computational and Structural Biotechnology Journal., 1(1), e201204005.
Clynes, R. A., Towers, T. L., Presta, L. G., & Ravetch, J. V. (2000). Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Medicine., 6(4), 443–446.
Danforth, D. N., Jr. (2016). Genomic changes in normal breast tissue in women at normal risk or at high risk for breast cancer. Breast Cancer: Basic and Clinical Research, 10, BCBCR-S39384.
Davis, P., & Howard, A. (2019). Chemotherapy and hormonal therapy. Core Curriculum for Oncology Nursing E-Book. 227.
Dhyani, P., Quispe, C., Sharma, E., Bahukhandi, A., Sati, P., Attri, D. C., et al. (2022). Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell International., 22(1), 1–20.
Erkko, H., Xia, B., Nikkilä, J., Schleutker, J., Syrjäkoski, K., Mannermaa, A., et al. (2007). A recurrent mutation in PALB2 in Finnish cancer families. Nature, 446(7133), 316–319.
Esplin, E. D., & Snyder, M. P. (2014). Genomic era diagnosis and management of hereditary and sporadic colon cancer. World Journal of Clinical Oncology., 5(5), 1036.
Fackenthal, J. D., & Olopade, O. I. (2007). Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nature Reviews Cancer., 7(12), 937–948.
Fang, D. D., Tao, R., Wang, G., Li, Y., Zhang, K., Xu, C., et al. (2022). Discovery of a novel ALK/ROS1/FAK inhibitor, APG-2449, in preclinical non-small cell lung cancer and ovarian cancer models. BMC Cancer, 22(1), 1–15.
Faraji, A., Dehghan Manshadi, H. R., Mobaraki, M., Zare, M., & Houshmand, M. (2016). Association of ABCB1 and SLC22A16 Gene polymorphisms with incidence of doxorubicin-induced febrile neutropenia a survey of iranian breast cancer patients. PLoS One., 11(12), e0168519. https://doi.org/10.1371/journal.pone.0168519
Friedlander, P., Haupt, Y., Prives, C., & Oren, M. (1996). A mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Molecular and Cellular Biology, 16(9), 4961–4971.
Galiatsatos, P., & Foulkes, W. D. (2006). Familial adenomatous polyposis. Official Journal of the American College of Gastroenterology ACG., 101(2), 385–398.
Geisler, S., Børresen-Dale, A. L., Johnsen, H., Aas, T., Geisler, J., Akslen, L. A., et al. (2003). TP53 gene mutations predict the response to neoadjuvant treatment with 5-fluorouracil and mitomycin in locally advanced breast cancer. Clinical Cancer Research., 9(15), 5582–5588.
Gennari, R., Menard, S., Fagnoni, F., Ponchio, L., Scelsi, M., Tagliabue, E., et al. (2004). Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clinical Cancer Research., 10(17), 5650–5655.
Gesmundo, I., Pedrolli, F., Vitale, N., Bertoldo, A., Orlando, G., Banfi, D., et al. (2022). Antagonist of growth hormone-releasing hormone potentiates the antitumor effect of pemetrexed and cisplatin in pleural mesothelioma. International Journal of Molecular Sciences., 23(19), 11248.
Ghosh, S., & Ghosh, A. (2021). Activation of DNA damage response signaling in mammalian cells by ionizing radiation. Free Radical Research., 55(8), 814–827.
Ghouse, M. S. (2020). An overview on plant derived anticancer drugs. Research Journal of Pharmacognosy and Phytochemistry., 12(4), 235–244.
Ginsburg, O., Yip, C. H., Brooks, A., Cabanes, A., Caleffi, M., Dunstan Yataco, J. A., et al. (2020). Breast cancer early detection: A phased approach to implementation. Cancer, 126, 2379–2393.
Glasgow, M. D., & Chougule, M. B. (2015). Recent developments in active tumor targeted multifunctional nanoparticles for combination chemotherapy in cancer treatment and imaging. Journal of Biomedical Nanotechnology., 11(11), 1859–1898.
Godet, I., & Gilkes, D.M. (2017). BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integrative cancer science and therapeutics. 4(1).
Hensel, M., Schneeweiss, A., Sinn, H. P., Egerer, G., Solomayer, E., Haas, R., et al. (2002). P53 is the strongest predictor of survival in high-risk primary breast cancer patients undergoing high-dose chemotherapy with autologous blood stem cell support. International Journal of Cancer., 100(3), 290–296.
Herr, I., & Debatin, K. M. (2001). Cellular stress response and apoptosis in cancer therapy. Blood, the Journal of the American Society of Hematology., 98(9), 2603–2614.
Hess, K. R., Anderson, K., Symmans, W. F., Valero, V., Ibrahim, N., Mejia, J. A., et al. (2006). Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. Journal of Clinical Oncology., 24(26), 4236–4244.
Holbro, T., Beerli, R. R., Maurer, F., Koziczak, M., Barbas, C. F., III., & Hynes, N. E. (2003). The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proceedings of the National Academy of Sciences., 100(15), 8933–8938.
Hynes, N. E., & Lane, H. A. (2005). ERBB receptors and cancer: The complexity of targeted inhibitors. Nature Reviews Cancer., 5(5), 341–354.
Junttila, T. T., Akita, R. W., Parsons, K., Fields, C., Phillips, G. D. L., Friedman, L. S., et al. (2009). Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell, 15(5), 429–440.
Karami, F., & Mehdipour, P. (2013). A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. BioMed research international. 2013.
Karampelas, I., & Sloan, A. E. (2018). Laser-induced interstitial thermotherapy of gliomas. Intracranial Gliomas Part III-Innovative Treatment Modalities., 32, 14–26.
Kaur, S., Kaur, J., Sharma, K., Bhadariya, V., & Singh, J. (2022). A review on the role of nutrition in combating cancer.
Kenneth, B.P. (2017). Infections caused by oncovirinae. In Handbook of Zoonoses. CRC Press. p. 511–5.
Kim, K.W., Roh, J.K., Wee, H.J., & Kim, C. (2016). Alkylating anticancer drugs. In Cancer drug discovery. Springer. p. 71–94.
Kim, M. J., Huang, Y., & Park, J. I. (2020). Targeting wnt signaling for gastrointestinal cancer therapy: Present and evolving views. Cancers, 12(12), 3638.
Kim, W. T., & Ryu, C. J. (2017). Cancer stem cell surface markers on normal stem cells. BMB Reports., 50(6), 285.
Kim, Y., Park, J., Joo, S. Y., Kim, B. G., Jo, A., Lee, H., et al. (2022). Structure of the Human ℡O2-TTI1-TTI2 Complex. Journal of Molecular Biology., 434(2), 167370.
Kis, A.M., Macasoi, I., Paul, C., Radulescu, M., Buzatu, R., & Watz, C.G. et al. (2022) Methotrexate and Cetuximab—Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina [Internet]. 58(2). Available from: https://www.mdpi.com/1648-9144/58/2/167
Kovalev, I. S., Zyryanov, G. V., Santra, S., Majee, A., Varaksin, M. V., & Charushin, V. N. (2022). Folic Acid Antimetabolites (Antifolates): A Brief Review on Synthetic Strategies and Application Opportunities. Molecules, 27(19), 6229.
Kröger, N., Milde-Langosch, K., Riethdorf, S., Schmoor, C., Schumacher, M., Zander, A. R., et al. (2006). Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clinical Cancer Research., 12(1), 159–168.
Lajous, H., Lelièvre, B., Vauléon, E., Lecomte, P., & Garcion, E. (2019). Rethinking alkylating (-like) agents for solid tumor management. Trends in Pharmacological Sciences., 40(5), 342–357.
Lanka, N. R., & Pulicherla, K. K. (2018). Pediatric hematological malignacies-clinical manifestation, treatment and follow-up. Frontiers in Clinical Drug Research: Hematology., 3(4), 138–183.
Lee-Hoeflich, S. T., Crocker, L., Yao, E., Pham, T., Munroe, X., Hoeflich, K. P., et al. (2008). A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Research., 68(14), 5878–5887.
Liu, H., & Zhao, C.(2022). Research progress on classification, characteristics and mechanism of antitumor drugs. In: AIP Conference Proceedings. AIP Publishing LLC. p. 020053.
Liu, J., Hong, S., Yang, J., Zhang, X., Wang, Y., Wang, H., et al. (2022). Targeting purine metabolism in ovarian cancer. Journal of Ovarian Research., 15(1), 1–22.
Lutz, S., Balboni, T., Jones, J., Lo, S., Petit, J., Rich, S. E., et al. (2017). Palliative radiation therapy for bone metastases: Update of an ASTRO evidence-based guideline. Practical Radiation Oncology., 7(1), 4–12.
Maguire, L. H., Thomas, A. R., & Goldstein, A. M. (2015). Tumors of the neural crest: Common themes in development and cancer. Developmental Dynamics., 244(3), 311–322.
Malamou-Mitsi, V., Gogas, H., Dafni, U., Bourli, A., Fillipidis, T., Sotiropoulou, M., et al. (2006). Evaluation of the prognostic and predictive value of p53 and Bcl-2 in breast cancer patients participating in a randomized study with dose-dense sequential adjuvant chemotherapy. Annals of Oncology., 17(10), 1504–1511.
Maoz, A., Matsuo, K., Ciccone, M. A., Matsuzaki, S., Klar, M., Roman, L. D., et al. (2020). Molecular pathways and targeted therapies for malignant ovarian germ cell tumors and sex cord-stromal tumors: A contemporary review. Cancers, 12(6), 1398.
Marches, R., & Uhr, J. W. (2004). Enhancement of the p27Kip1-mediated antiproliferative effect of trastuzumab (Herceptin) on HER2-overexpressing tumor cells. International Journal of Cancer., 112(3), 492–501.
Maringe, C., Spicer, J., Morris, M., Purushotham, A., Nolte, E., Sullivan, R., et al. (2020). The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. The Lancet Oncology., 21(8), 1023–1034.
Menard, S., Pupa, S. M., Campiglio, M., & Tagliabue, E. (2003). Biologic and therapeutic role of HER2 in cancer. Oncogene, 22(42), 6570–6578.
Miki, Y., Swensen, J., Shattuck-Eidens, D., Futreal, P. A., Harshman, K., Tavtigian, S., et al. (1994). A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science, 266(5182), 66–71.
Mohammed, A. Y., & Ahamed, L. S. (2022). Synthesis and characterization of new substituted coumarin derivatives and study their biological activity. Chem Methodol., 6, 813–822.
Mori, T., Nagase, H., Horii, A., Miyoshi, Y., Nakatsuru, S., Aoki, T., et al. (1994). Germ-line and somatic mutations of the APC gene in patients with Turcot syndrome and analysis of APC mutations in brain tumors. Genes, Chromosomes and Cancer., 9(3), 168–172.
Motegi, A., Fujimoto, J., Kotani, M., Sakuraba, H., & Yamamoto, T. (2004). ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. Journal of Cell Science., 117(15), 3319–3329.
Moynahan, M. E. (2002). The cancer connection: BRCA1 and BRCA2 tumor suppression in mice and humans. Oncogene, 21(58), 8994–9007.
Naito, Y., Takahashi, H., Shitara, K., Okamoto, W., Bando, H., Kuwata, T., et al. (2018). Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Japanese Journal of Clinical Oncology., 48(6), 559–564.
Namazi, H., Kulish, V. V., & Wong, A. (2015). Mathematical modelling and prediction of the effect of chemotherapy on cancer cells. Scientific Reports, 5(1), 1–8.
Nour-Eldin, N. E. A., Exner, S., Al-Subhi, M., Naguib, N. N., Kaltenbach, B., Roman, A., et al. (2017). Ablation therapy of non-colorectal cancer lung metastases: Retrospective analysis of tumour response post-laser-induced interstitial thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA). International Journal of Hyperthermia., 33(7), 820–829.
Ohashi, S., Miyamoto, S., Kikuchi, O., Goto, T., Amanuma, Y., & Muto, M. (2015). Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology, 149(7), 1700–1715.
Olivier, M., Hollstein, M., & Hainaut, P. (2010). TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harbor Perspectives in Biology., 2(1), a001008.
Olivier, M., Langer√∏d, A., Carrieri, P., Bergh, J., Klaar, S., Eyfjord, J., et al. (2006). The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research., 12(4), 1157–1167.
Paraf, F., Jothy, S., & Van Meir, E. G. (1997). Brain tumor-polyposis syndrome: Two genetic diseases? Journal of Clinical Oncology., 15(7), 2744–2758.
Peart, O. (2017). Metastatic breast cancer. Radiologic Technology., 88(5), 519M-539M.
Peng, M., Darko, K. O., Tao, T., Huang, Y., Su, Q., He, C., et al. (2017). Combination of metformin with chemotherapeutic drugs via different molecular mechanisms. Cancer Treatment Reviews., 54, 24–33.
Pérez-Herrero, E., & Fernández-Medarde, A. (2015). Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. European Journal of Pharmaceutics and Biopharmaceutics., 93, 52–79.
Petitjean, A., Mathe, E., Kato, S., Ishioka, C., Tavtigian, S. V., Hainaut, P., et al. (2007). Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Human Mutation., 28(6), 622–629.
Pierce, J. H., Arnstein, P., DiMarco, E., Artrip, J., Kraus, M. H., Lonardo, F., et al. (1991). Oncogenic potential of erbB-2 in human mammary epithelial cells. Oncogene, 6(7), 1189–1194.
Priestman, T. (2008). Some practical aspects of cancer chemotherapy. In Cancer chemotherapy in clinical practice. Springer. p. 35–77.
Rahko, E., Blanco, G., Soini, Y., Bloigu, R., & Jukkola, A. (2003). A mutant TP53 gene status is associated with a poor prognosis and anthracycline-resistance in breast cancer patients. European Journal of Cancer., 39(4), 447–453.
Rao, C. V., & Yamada, H. Y. (2013). Genomic instability and colon carcinogenesis: From the perspective of genes. Frontiers in Oncology., 3, 130.
Reshetnyak, A. V., Mohanty, J., Tomé, F., Puleo, D. E., Plotnikov, A. N., Ahmed, M., et al. (2018). Identification of a biologically active fragment of ALK and LTK-Ligand 2 (augmentor-α). Proceedings of the National Academy of Sciences., 115(33), 8340–8345.
Rouzier, R., Perou, C. M., Symmans, W. F., Ibrahim, N., Cristofanilli, M., Anderson, K., et al. (2005). Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clinical Cancer Research., 11(16), 5678–5685.
Rowan, A. J., Lamlum, H., Ilyas, M., Wheeler, J., Straub, J., Papadopoulou, A., et al. (2000). APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits.” Proceedings of the National Academy of Sciences., 97(7), 3352–3357.
Rowan, S., Ludwig, R. L., Haupt, Y., Bates, S., Lu, X., Oren, M., et al. (1996). Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. The EMBO Journal., 15(4), 827–838.
Rubin, I., & Yarden, Y. (2001). The basic biology of HER2. Annals of Oncology., 12, S3-8.
Rubinfeld, B., Souza, B., Albert, I., Müller, O., Chamberlain, S. H., Masiarz, F. R., et al. (1993). Association of the APC gene product with β-catenin. Science, 262(5140), 1731–1734.
Sakanaka, C., Weiss, J. B., & Williams, L. T. (1998). Bridging of β-catenin and glycogen synthase kinase-3β by axin and inhibition of β-catenin-mediated transcription. Proceedings of the National Academy of Sciences., 95(6), 3020–3023.
Sallman, D. A., McLemore, A. F., Aldrich, A. L., Komrokji, R. S., McGraw, K. L., Dhawan, A., et al. (2020). TP53 mutations in myelodysplastic syndromes and secondary AML confer an immunosuppressive phenotype. Blood, 136(24), 2812–2823.
Semalatha, S. (2018). Scientific Validation of Anti-cancer, Anti tumour and Anti-oxidant activities of Siddha Herbo-mineral Formulation Bhramasthiram in Various cell lines studies [PhD Thesis]. Government Siddha Medical College, Chennai.
Shaw, A. T., & Engelman, J. A. (2013). ALK in lung cancer: Past, present, and future. Journal of Clinical Oncology., 31(8), 1105.
Sibilio, A., Curcio, A., Toesca, A., Rossi, E. M. C., & Corso, G. (2022). Local treatment in patients with hereditary breast cancer: Decision-making process in low-, moderate-, high-penetrance pathogenic germline mutation carriers. Current Opinion in Oncology., 34(6), 614–622.
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians, 71(1), 7–33.
Siegel, R. L., Miller, K. D., & Jemal, A. (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34.
Smith, M. J., Neel, B. G., & Ikura, M. (2013). NMR-based functional profiling of RASopathies and oncogenic RAS mutations. Proceedings of the National Academy of Sciences., 110(12), 4574–4579.
Smits, R., Ruiz, P., Diaz-Cano, S., Luz, A., Jagmohan-Changur, S., Breukel, C., et al. (2000). E-cadherin and adenomatous polyposis coli mutations are synergistic in intestinal tumor initiation in mice. Gastroenterology, 119(4), 1045–1053.
Solyom S. (2011). BRCA/FANCONI Anemia Pathway Genes in Hereditary Predisposition to Breast Cancer. University of Oulu.
Song, Z., Wu, Y., Yang, J., Yang, D., & Fang, X. (2017). Progress in the treatment of advanced gastric cancer. Tumor Biology., 39(7), 1010428317714626.
Soussi, T. (2007). p53 alterations in human cancer: More questions than answers. Oncogene, 26(15), 2145–2156.
Southey, M. C., Winship, I., & Nguyen-Dumont, T. (2016). PALB2: Research reaching to clinical outcomes for women with breast cancer. Hereditary Cancer in Clinical Practice., 14(1), 1–7.
Souttou, S., Benabdesselam, R., Siqueiros-Marquez, L., Sifi, M., Deliba, M., Vacca, O., et al. (2019). Expression and localization of dystrophins and β-dystroglycan in the hypothalamic supraoptic nuclei of rat from birth to adulthood. Acta Histochemica., 121(2), 218–226.
Sparks, A. B., Morin, P. J., Vogelstein, B., & Kinzler, K. W. (1998). Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Research., 58(6), 1130–1134.
Spirio, L. N., Samowitz, W., Robertson, J., Robertson, M., Burt, R. W., & Leppert, M. (1998). Alleles of APC modulate the frequency and classes of mutations that lead to colon polyps. Nature Genetics., 20(4), 385–388.
Stephens, F.O., & Aigner, K.R. (2016). Symptoms of cancer: local and general. In: Basics of Oncology. Springer. p. 47–52.
Stone, T. W., McPherson, M., & Darlington, L. G. (2018). Obesity and cancer: Existing and new hypotheses for a causal connection. eBioMedicine, 30, 14–28.
Tabernero, J., Grothey, A., Arnold, D., de Gramont, A., Ducreux, M., O’Dwyer, P., et al. (2022). MODUL cohort 2: An adaptable, randomized, signal-seeking trial of fluoropyrimidine plus bevacizumab with or without atezolizumab maintenance therapy for BRAFwt metastatic colorectal cancer. ESMO Open., 7(5), 100559.
Takamura, Y., Kobayashi, H., Taguchi, T., Motomura, K., Inaji, H., & Noguchi, S. (2002). Prediction of chemotherapeutic response by collagen gel droplet embedded culture-drug sensitivity test in human breast cancers. International Journal of Cancer., 98(3), 450–455.
Thakur, C., Nayak, P., Mishra, V., Sharma, M., & Saraogi, G.K. (2021). Treating blood cancer with nanotechnology: a paradigm shift. In Nano Drug Delivery Strategies for the Treatment of Cancers. Elsevier. p. 225–43.
Thompson, D., Easton, D., Consortium BCL. (2002). Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiology Biomarkers & Prevention., 11(4), 329–336.
Thompson, D., & Easton, D. (2004). The genetic epidemiology of breast cancer genes. Journal of Mammary Gland Biology and Neoplasia, 9(3), 221–236.
Topi, S., Santacroce, L., Bottalico, L., Ballini, A., Inchingolo, A. D., Dipalma, G., et al. (2020). Gastric cancer in history: A perspective interdisciplinary study. Cancers, 12(2), 264.
Tudyka, V. N., & Clark, S. K. (2012). Surgical treatment in familial adenomatous polyposis. Annals of Gastroenterology., 25(3), 201.
Tung, C. L., Chao, W. Y., Li, Y. Z., Shen, C. H., Zhao, P. W., Chen, S. H., et al. (2022). Ivermectin induces cell cycle arrest and caspase-dependent apoptosis in human urothelial carcinoma cells. International Journal of Medical Sciences., 19(10), 1567–1575.
Turpin, E., Bieche, I., Bertheau, P., Plassa, L. F., Lerebours, F., de Roquancourt, A., et al. (2002). Increased incidence of ERBB2 overexpression and TP53 mutation in inflammatory breast cancer. Oncogene, 21(49), 7593–7597.
Uljanova, O.V., Ogneva, A.S. (2021). Classification of oncological diseases in pets. In Aктyaльныe пpoблeмы aгpoпpoмышлeннoгo кoмплeкca. p. 931–4.
Varricchi, G., Ameri, P., Cadeddu, C., Ghigo, A., Madonna, R., Marone, G., et al. (2018). Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Frontiers in Physiology, 9, 167.
Ventola, C. L. (2017). Cancer immunotherapy, part 1: Current strategies and agents. Pharmacy and Therapeutics., 42(6), 375.
Vousden, K. H., & Lu, X. (2002). Live or let die: The cell’s response to p53. Nature Reviews Cancer., 2(8), 594–604.
Walsh, T., Mandell, J. B., Norquist, B. M., Casadei, S., Gulsuner, S., Lee, M. K., et al. (2017). Genetic predisposition to breast cancer due to mutations other than BRCA1 and BRCA2 founder alleles among Ashkenazi Jewish women. JAMA Oncology., 3(12), 1647–1653.
Wendt, C., & Margolin, S. (2019). Identifying breast cancer susceptibility genes–a review of the genetic background in familial breast cancer. Acta Oncologica., 58(2), 135–146.
Willis, A., Jung, E. J., Wakefield, T., & Chen, X. (2004). Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene, 23(13), 2330–2338.
Wooster, R., Bignell, G., Lancaster, J., Swift, S., Seal, S., Mangion, J., et al. (1995). Identification of the breast cancer susceptibility gene BRCA2. Nature, 378(6559), 789–792.
Xia, B., Dorsman, J. C., Ameziane, N., De Vries, Y., Rooimans, M. A., Sheng, Q., et al. (2007). Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nature Genetics., 39(2), 159–161.
Xia, M. X., Shi, Z. M., Xing, L., Gao, D. J., Ye, X., Wang, T. T., et al. (2022). Endoscopic radiofrequency ablation may improve overall survival in patients with inoperable ampullary carcinoma. Digestive Endoscopy., 34(3), 587–595.
Xing, P. Y., Zhu, Y. X., Wang, L., Hui, Z. G., Liu, S. M., Ren, J. S., et al. (2019). What are the clinical symptoms and physical signs for non-small cell lung cancer before diagnosis is made? a nation-wide multicenter 10-year retrospective study in China. Cancer Medicine., 8(8), 4055–4069.
Xu, C., Zhu, S., Wu, M., Han, W., & Yu, Y. (2014). Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN). Biological and Pharmaceutical Bulletin., 37(4), 511–520.
Xu, J., Elshazly, A. M., & Gewirtz, D. A. (2022). The cytoprotective, cytotoxic and nonprotective functional forms of autophagy induced by microtubule poisons in tumor cells—implications for autophagy modulation as a therapeutic strategy. Biomedicines., 10(7), 1632.
Yakes, F. M., Chinratanalab, W., Ritter, C. A., King, W., Seelig, S., & Arteaga, C. L. (2002). Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Research., 62(14), 4132–4141.
Zhang, K., Sun, J., Liu, N., Wen, D., Chang, D., Thomason, A., et al. (1996). Transformation of NIH 3T3 Cells by HER3 or HER4 Receptors Requires the Presence of HER1 or HER2 (∗). Journal of Biological Chemistry., 271(7), 3884–3890.
Acknowledgements
We would like to express our sincere gratitude to Areo Stephen and Priscilla Bakare for providing valuable insight and suggestions during the preparation of the graphical abstract for this publication. Their expertise and guidance have been invaluable in creating a visually appealing and informative summary of our research. We would also like to extend our appreciation to Alalade Daniel and Ogunshina Theophilus for their exceptional skills in designing the abstract and images for this publication. His attention to detail and creativity have resulted in a clear and concise summary that effectively captures the essence of our work.
Funding
Funding (information that explains whether and by whom the research was supported).
Author information
Authors and Affiliations
Department of Biology, San Diego State University, San Diego, USA
Adekunle Fiyin Ademikanra
Department of Biochemistry, Ladoke Akintola University of Technology, Ogbomosho, Nigeria
Olutayo Micheal Oyewole
Department of Microbiology, Federal University Oye-Ekiti, Oye-Ekit, Nigeria
Azeemat Olanrewaju Olayiwola
Contributions
AA performed the literature review on common hotspots of cancer chemotherapy and contributed to the writing of the manuscript. OO collected and analyzed data on the efficacy of chemotherapy on different cancer types. OA provided insight on the side effects and management of chemotherapy in various cancer cases and contributed to the writing of the manuscript. All authors read and approved the final manuscript. Studies involving plants must include a statement specifying the local, national, or international guidelines and legislation and the required or appropriate permissions and/or licenses for the study: not applicable.
Corresponding author
Correspondence to Adekunle Fiyin Ademikanra.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 59 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ademikanra, A.F., Oyewole, O.M. & Olayiwola, A.O. Common hotspots of cancer chemotherapy. GENOME INSTAB. DIS. 4, 181–196 (2023). https://doi.org/10.1007/s42764-023-00101-9
Received18 February 2023
Revised18 May 2023
Accepted21 May 2023
Published13 June 2023
Issue DateJune 2023
DOIhttps://doi.org/10.1007/s42764-023-00101-9
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable linkKeywords
Cancer
Carcinogenesis
Carcinoma
Sarcoma
Blastoma
Lymphoma
Leukemia
Oncogenes
Chemotherapy








用户登录
还没有账号?
立即注册