Novel insights into DNA damage repair defects in HPV-positive head and neck squamous cell carcinoma: from the molecular basis to therapeutic opportunities
Review Article
Qi Liu, Nan Zuo, Xinghan Li, Yongqiang Deng, Lanlan Wei & Lin Ma
Genome Instability & Disease 4, 255–265 (2023)
Abstract
The incidence of human papillomavirus (HPV) associated head and neck squamous cell carcinoma (HNSCC) has dramatically increased in recent decades. There is clear evidence in literature that patients with HPV-positive HNSCC have a significantly better prognosis after genotoxic therapies compared to those with HPV-negative HNSCC. This favorable outcome has been associated with distinct features in DNA damage repair (DDR) functions specific to HPV-positive HNSCC. As a result, weaknesses in DDR have been identified for both HPV-positive and HPV-negative HNSCC, respectively. Therefore, personalized therapy targeting the vulnerable aspects of DDR pathways based on HPV status and mutational profiles has been proposed for precise treatment of HNSCC patients. This review focuses on the most recent evidence regarding the impact of HPV on DDR pathways and the related therapeutic strategies in HNSCC. We anticipate that future translation of these discoveries into clinical practice may lead to treatment de-escalation approaches in HPV-positive HNSCC and more effective therapies for the poor prognosis HPV-negative HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a class of malignant epithelial tumors that mainly affect the oral cavity, the pharynx, and the larynx (Leemans et al., 2011). According to the latest epidemiological study published in 2021, HNSCC affects approximately 870,000 people and causes more than 440,000 deaths worldwide annually (Sung et al., 2021). Besides tobacco and alcohol, human papillomavirus (HPV) infection is another important risk factor for HNSCC (de Martel et al., 2017). Over the past two decades, the annual incidence of HPV-associated HNSCC has dramatically increased worldwide. One study has reported a 36.5% increase in the global incidence of HPV-associated HNSCC (Sung et al., 2021). Oropharyngeal cancer has the highest HPV detection rate (~ 22.4%) among HPV-associated HNSCC cases, followed by oral cancer (~ 4.4%) and laryngeal cancer (~ 1.5%) (Castellsague et al., 2016). In addition, the HPV infection rate in HNSCC is relatively higher in developed countries and regions such as Europe, America, Australia, New Zealand, and Japan (de Martel et al., 2017).
HPV is a DNA virus with a double-stranded circular genome of approximately 8000 bp (Doorbar et al., 2012). More than 400 HPV subtypes have been identified to date. HPVs are classified into high- and low-risk types based on their cancer-causing potential (McBride, 2022), whereas through this review HPV generally refers to high-risk HPV. Among the high-risk HPV types, HPV16/18 has the strongest association with cancer development, as reported in 71% of the cervical cancers, and 85% of HPV-associated HNSCC (de Martel et al., 2017). The HPV genome is mainly composed of the early (E) and late (L) genes. Early genes, including E1, E2, E4, E5, E6, E7, and E8, are responsible for viral replication and cellular transformation (McBride, 2022). Late genes, including L1 and L2, are responsible for viral assembly. High-risk HPV is mainly carcinogenic via E6 and E7, which inhibits the expression of certain cellular proteins. For example, E6 induces the degradation of the tumor suppressor p53, leading to cell cycle dysregulation and diminished pro-apoptotic capacity (Tomaic, 2016). E7 binds to and inactivates pRb, releasing the transcription factor E2F, which disrupts the cell cycle and leads to aberrant cell proliferation and malignant transformation (Tomaic, 2016). Increasing evidence suggests E5 is another important viral oncogene in HPV-induced carcinogenesis (Basukala & Banks, 2021; Maufort et al., 2010).
Although HNSCC involves a heterogeneous group of tumors, HNSCC patients are commonly treated with radiotherapy or radiochemotherapy (Keam et al., 2021). These therapies use genotoxic agents that cause various types of DNA damages, including single-stranded DNA damage, interstrand crosslinks (ICL), and double-stranded breaks (DSB). An imbalance between DNA damage and repair results in the death of tumor cells and the suppression of tumor growth. Mounting evidence has revealed that HPV-positive HNSCC responds significantly better to genotoxic treatment than HPV-negative HNSCC, which has been associated with deficient functions in DNA damage repair (DDR) (Ang et al., 2010; Liu et al., 2018a, 2018b; Zech et al., 2022). Thus, de-escalation treatment of radiochemotherapy for HPV-positive HNSCC has been proposed and tested in clinical trials, although no internationally accredited clinical consensus has been approved yet. To achieve a precise treatment for HNSCC, the mechanisms underlying the impact of HPV on DDR must be thoroughly elucidated.
DNA damage repair pathways in HNSCC
Like all other mammalian cells, HNSCC cells possess several DDR mechanisms for genomic stability. According to the repair characteristics, they can be broadly summarized as excision repair, mismatch repair (MMR), ICL repair, and DSB repair (Fig. 1).
Fig. 1
Simplified schematic summary of excision repair, mismatch repair, ICL repair and DSB repair pathways. The genes in the blue box represent typical regulatory genes of the corresponding pathway
Excision repair includes base excision repair (BER) and nucleotide excision repair (NER). BER is a highly conserved mechanism for repairing a wide variety of DNA lesions caused by endogenous or exogenous sources, including deamidation, depurination, alkylation, oxidative damage, repair of small base deletions, and removal of small fragments of DNA from adducts with other macromolecules (Grundy & Parsons, 2020). The NER pathway is widely used in mammalian cells and excels in repairing helix-distorting lesions caused by chemotherapeutic agents, particularly ultraviolet (UV) photoproducts and massive covalent adducts.
MMR is a highly conserved biological pathway that plays a key role in maintaining genomic stability (Li., 2008). During DNA replication, DNA polymerases introduce base mismatches or small insertions/deletions by chance, and MMR is responsible for correcting such errors (Fishel, 2015). MMR is also involved in the repair of DNA damage caused by exogenous stresses such as cisplatin, free radical oxygen, and UV.
ICL is a form of DNA damage in which bases are covalently cross-linked between DNA double-strands. ICL can be triggered by endogenous active aldehydes as well as by natural compounds such as psoralen, mitomycin C (MMC), and the clinically used chemotherapeutic agent cisplatin (Deans & West, 2011). Repair of ICL primarily relies on the Fanconi anemia (FA) pathway with a network of at least 22 genes (Zuo et al., 2023). A key step in the FA pathway is the monoubiquitination of FANCD2 and FANCI, which is facilitated by a large E3 ubiquitin ligase complex including FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, FANCM, FANCT, and FANCI. The FA core complex in combination with downstream FA proteins (including FANCP, XPF, BRCA1/2, FANCU, FANCJ, FANCO, FANCN, FANCW, FANCS, FANV) cooperate with BER, NER, and DSB pathways to complete DDR. The DNA structure-specific nucleic acid endonucleases ERCC1-XPF, MUS81-EME1, and SLX1 play an important role in cleavage of cross-linked DNA at stalled replication forks in a process known as unhooking. The DSB substrate forms after excision of cross-linked DNA by endonucleases, and the DSB repair pathways are subsequently recruited for completion of ICL repair.
The DSB repair pathway predominantly involves homologous recombination (HR), non-homologous end-joining (NHEJ), and alternative end-joining (alt-EJ) (Liu et al., 2019). The HR pathway is a faultless repair mode that employs homologous sister chromatids for repairing DSB. Thus, HR occurs mainly in the G2/M and late S phases of the cell cycle. HR primarily takes the following steps: (1) MRN (MRE11–RAD51–NBS1) complex localizes to the DSB and process it with CtIP, EXO1, etc. to form 3′ end single-stranded DNA (ssDNA). ssDNA is further wrapped by replication protein A (RPA) to avoid degradation by nuclease digestion. (2) BRCA2 is activated and binds to RAD51 recombinase, replacing RPA on the ssDNA, which results in RAD51-associated nucleoprotein filaments and invade the DNA strand structure, searching for homologous sequences on sister chromatids. (3) DNA polymerase replicates the missing DNA fragment from the homologous template. Then, the crossover migrates to form the Holiday junction structure. (4) Holiday junction dissociates, and DNA ligase joins the broken ends of DNA to complete DSB repair.
NHEJ occurs throughout the cell cycle and functions predominantly during the G0/G1 phase (Liu et al., 2019). At the beginning of NHEJ, tumor suppressor p53-binding protein 1 (53BP1) is recruited to DSB and interacts with Ku70/80 heterodimers to co-stabilize the broken DNA ends. Subsequently, the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) interacts with the Ku70/80 heterodimer to deliver the repair signal. At this stage, Artemis, PNKP, etc. are recruited to modify and process the broken DNA ends, leading to minor nucleotide addition or deletion at the DSB sites. Finally, XRCC4-Lig IV ligase completes the final ligation of the DSB.
alt-EJ was initially identified in NHEJ-deficient cells and considered as an alternative repair pathway for rejoining DSB in the context of compromised NHEJ and HR. Because of the characteristic pairing of microhomology upstream and downstream of the DSB with 2–20 bp microhomologous sequences, alt-EJ is also known as microhomology-mediated end-joining (MMEJ). alt-EJ uses poly [ADP-ribose] polymerase 1 (PARP1) to detect DSB and stabilize the broken DNA ends, the CtIP-mediated MRN complex to generate 3′ ssDNA, EXO1 or BLM to further process the DNA ends when microhomologous sequences are far apart. After pairing of the microhomology in the opposite ssDNA and resection of the 3′ overhangs, DNA polymerase theta (POLQ) is used to tether the microhomology and complete DNA replication at the DSB sites within two single-stranded 3′ overhangs (Liu et al., 2019). Finally, the ligation of the DNA is completed by LIG I/III/IV. alt-EJ frequently results in DNA deletions, insertions, and gross chromosomal rearrangements, and closely associates with low DNA repair fidelity and high genomic instability. Notably, alt-EJ pathway is commonly upregulated in cancers, suggesting potential therapeutic targets involved in alt-EJ (Patterson-Fortin & D’Andrea, 2020).
HPV-induced DDR defects in HNSCC
Upon infection, HPV express viral oncoproteins to disrupt the function of tumor suppressor genes in host cells. Alternatively, it may integrate viral DNA into adjacent coding regions, resulting in the duplication or deletion of nearby introns, thereby promoting or inhibiting the expression of certain gene products. Notably, a study characterizing HPV integrations in 279 HNSCC reported that 60% of HPV integration breakpoints occurred in DNA regions with microhomology, suggesting alt-EJ is exploited during HPV induced carcinogenesis (Parfenov et al., 2014). Indeed, HPV usually hijack DDR genes to facilitate genomic instability and carcinogenesis.
HNSCC are commonly treated with genotoxic agents. The treatment outcome suggested that HPV-positive HNSCC have profound DDR defects. In a retrospective clinical study with 206 HPV-positive and 117 HPV-negative oropharyngeal cancer, combined treatment with radiotherapy and cisplatin resulted in significant better overall survival in HPV-positive patients (Ang et al., 2010). The powerful prognostic value of HPV has been confirmed in a number of following studies with hundreds of HNSCC patients (Lohaus et al., 2014). To reduce normal tissue toxicity, clinical tests of dose de-escalated radiotherapy have been performed for HPV-positive HNSCC (Gabani et al., 2019).
Deficient DDR increases radiosensitivity in cancer. An in vivo tumor growth delay study on patient-derived xenografts showed that two of three HPV-positive tumors were most sensitive to x-ray irradiation compared to the HPV-negative tumors (Lilja-Fischer et al., 2019). Although tumor microenvironmental factors such as hypoxia may play a role in the differential therapeutic responses, clonogenic cell survival studies on HNSCC cell lines in vitro (i.e., without microenvironmental components) also identified consistent higher radiosensitivity in HPV-positive HNSCC (Kimple et al., 2013; Liu et al., 2022a, 2022b), indicating that HPV-associated intrinsic DDR defects in cancer cells play a critical role in the better therapeutic response of HPV-positive HNSCC to genotoxic agents. Indeed, abundant evidence has demonstrated the importance of DDR deficiency in HPV-enhanced radiosensitivity (Fabbrizi & Parsons, 2020).
For chemotherapy, FA/BRCA pathway is required to repair cisplatin induced ICL. A few recent studies have revealed that HPV affect cellular sensitivity to cisplatin through impaired FA pathway. Overexpression of HPV E6 in human foreskin keratinocytes resulted in prolonged deubiquitination of FANCD2, thereby disrupting normal FA pathway function (Khanal & Galloway, 2019). Huang et al. found that HPV E6/E7 downregulated SERPINB3 in HNSCC, resulting in impaired FA pathway via the E6E7/SERPINB2/USP1/FANCD2-FANCI axis and increased cisplatin sensitivity in HPV-positive HNSCC. In addition, a recent study provided consistent results, confirming that HPV confers a FA-like phenotype in HNSCC via disrupted XPF function both in vitro and in vivo (Zuo et al., 2023).
DSB is the most dangerous type of DNA damage during genotoxic therapies. Therefore, the effects of HPV on DSB repair pathway have been extensively studied. Dysregulated HR has been commonly observed in HPV-positive HNSCC. Köcher et al. found that ataxia telangiectasia mutated (ATM) was suppressed in HPV-positive HNSCC cells (Kocher et al., 2022). These cells exhibited only a small increase in γH2AX foci, mild G2/M cell cycle arrest and slight cisplatin sensitization compared to HPV-negative HNSCC cells after ATM inhibitor treatment. In response to DSB, ATM is autophosphorylated at serine 1981 to promote DNA repair through HR. Consistently, Liu et al. found that HPV subjugation of TGFβ signaling suppressed ATM autophosphorylation through downregulation of FOXO3 (Liu et al., 2018a, 2018b). In addition, HPV-positive HNSCC cells showed fewer Rad51 foci, a specific biomarker for HR, after x-ray irradiation. These observations suggested that the inhibition of ATM expression or activation by HPV resulted in HR defects in HNSCC cells. Besides, more mechanisms underlying HPV-induced HR defects have been reported. Molkentine et al. found that HPV-induced p16 overexpression inhibits the ubiquitination of USP7 and TRIP12 by regulating SP1 and HUWE1, leading to HR defects in HPV-positive HNSCC cells (Molkentine et al., 2022). Dok et al. reported that p16INK4a overexpression led to a defect in HR by downregulating cyclin D1 protein expression and to a failure in localizing Rad51 to the site of DSB in HPV-positive HNSCC cells (Dok et al., 2014). Canonical NHEJ is the main pathway that rapidly detects and directly ligates the broken ends in DSB during genotoxic therapies. However, HPV may interfere the normal functions of NHEJ pathway in HNSCC. Alsahafi et al. found that EGFR overexpression in HPV-positive HNSCC cells downregulated HPV oncoprotein E6, reduced the expression of NHEJ-related proteins Ku80 and DNA-PKcs after radiotherapy, induced DDR defects and increased radiation response (Alsahafi et al., 2021). Similarly, Hu et al. observed that HPV promoted the destabilization of the transcript p300 in HNSCC cells, inhibited the expression of DNA-PKcs and affected the activity of NHEJ (Hu et al., 2020).
alt-EJ is primarily used in tumor cells to cope with DNA damages during DNA replication and genotoxic therapies where HR and NHEJ are truncated or saturated. A few studies have shown that HPV-positive cells have increased alt-EJ activity. Leeman et al. found that HPV oncoprotein E7 significantly increased the proportion of DNA deletions with flanking microhomology, a signature associated with MMEJ, in HNSCC cells, while canonical NHEJ was suppressed (Leeman et al., 2019). Liu et al. found that HPV inhibited the intracellular TGFβ signaling, resulting in suppressed expression of BRCA1 and FOXO3 through upregulation of miR-182, which was associated with increased expression of PARP1 and POLQ, resulting in enhanced activity of alt-EJ in HNSCC (Liu et al., 2018a, 2018b). Following this lead, the research team further established a panel of 36 genes that were related to alt-EJ, including PARP1 and POLQ, and found that 22 of them were significantly upregulated in HPV-positive HNSCC compared to the HPV-negative ones (Guix et al., 2022; Liu et al., 2021). Not only that, but the subclass of solid-tumor cancers, no matter what types of cancers they are, that exhibit similar biological features to HPV-positive HNSCC, i.e., low TGFβ signaling and high alt-EJ activity, were all more responsive to genotoxic therapies (Guix et al., 2022; Liu et al., 2021). Furthermore, the impaired FA pathway caused by HPV oncoproteins E6 and E7 also promoted greater dependence on alt-EJ to maintain genomic integrity in HNSCC cells (Zuo et al., 2023).
In summary, HPV affects normal functions and network system of multiple DNA repair pathways (Fig. 2). Considering the increased activity, alt-EJ is an ideal target for improved treatment of HPV-positive HNSCC. In addition, excision repair and MMR also play essential roles in tumor response to radiochemotherapy. However, there are relatively few studies concerned at the effect of HPV on these DDR pathways. Thus, further investigation on this topic is mandatory to completely understand HPV-associated DDR profiles in HNSCC.
Fig. 2
Schematic illustration of the HPV-induced effect on DNA damage repair in HNSCC. The genes in the blue box represent typical regulatory genes of the corresponding pathway. HPV inhibits the FA, HR, and NHEJ pathways’ activity in HNSCC from multiple angles. alt-EJ is the only upregulated DNA damage repair pathway in HPV-positive HNSCC
Targeting DDR for HNSCC treatment
Because DDR pathways profoundly affect cellular response to genotoxic agents, targeting DDR related genes has been extensively tested as a potential way to eradicate cancer cells, increase radiation sensitivity, and overcome cisplatin-resistance in HNSCC (Table 1). HPV-positive HNSCC showed distinct DDR profiles from HPV-negative cases. Thus, differential responses between HPV-positive and HPV-negative HNSCC to DDR inhibitors are expected. Precise application of DDR inhibitors will leverage the clinical effects of radiochemotherapy, achieve de-intensified treatment of HPV-positive HNSCC for less toxicity, and result in improved therapeutic outcomes in HPV-negative HNSCC.
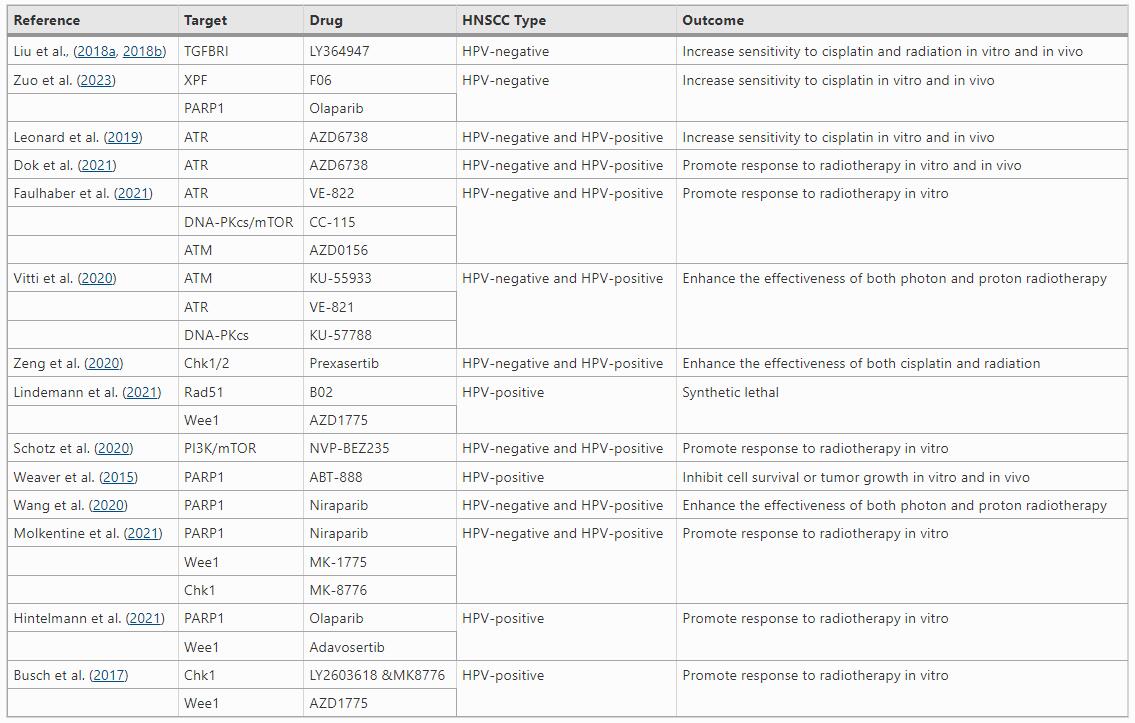
Table 1 Drugs targeting DNA damage repair in HNSCC
Various types of kinase inhibitors have been approved for clinical applications in the past decades, while some of these inhibitors have demonstrated impressive effectiveness in treating HNSCC (Kitamura et al., 2020; Wu et al., 2015). DDR pathways contains multiple kinases such as ATM, DNA-PKcs, ataxia telangiectasia and Rad3-related protein (ATR). ATR plays an important role in repair of cisplatin-induced DNA damage. Consistently, inhibiting ATR with small-molecule inhibitor AZD6738 enhanced cisplatin-induced DNA damage and apoptosis, thereby suppressing the growth of HPV-negative and HPV-positive HNSCC cells in vitro and in vivo (Leonard et al., 2019). ATR, ATM, and dual DNA-PK/mTOR inhibitors can promote radiosensitivity in photon/proton irradiation of cells and xenografts of HPV-negative and HPV-positive HNSCC (Dok et al., 2021; Faulhaber et al., 2021; Vitti et al., 2020).
ATR and ATM phosphorylate and activate checkpoint kinases 1 and 2 (CHK1/2), respectively. CHK1 and CHK2 are serine/threonine kinases that regulate cell cycle checkpoints when encountering DNA damage, leading to halt of cell cycle progression for DDR. Prexasertib, an inhibitor of CHK1/2, induced persistent DNA damage and increased apoptosis in HPV-positive and HPV-negative HNSCC cells treated with cisplatin and radiation, whereas little normal tissue toxicity was observed in mouse tumor models (Zeng et al., 2020). Moreover, HPV increases replication stress in HNSCC, which triggers ATR/CHK1 signaling pathway, leading to efficient DNA damage response for cell survival and proliferation. Thus, inhibition of the ATR/CHK1 pathway is a promising strategy for treating HPV-positive HNSCC (Karukonda et al., 2022).
Wee1 kinase and Rad51 recombinase are two crucial proteins involved in regulating replication stress and HR repair in cancer cells. Combined inhibition of both proteins achieved synergistic effects on HNSCC cell lines. The synergy between the two inhibitors was associated with forced CDK1 activation and reduced CHK1 phosphorylation leading to induction of excessive DNA damage and replication stress, culminating in aberrant mitosis and apoptosis (Lindemann et al., 2021). Consistent with these results, the combination of Rad51 and Wee1 inhibitors significantly inhibited tumor growth in vivo in mice bearing HPV-positive HNSCC tumors as compared to HPV-negative HNSCC. This differential sensitivity appears to be linked to the phenomenon that HPV-positive tumors have more endogenous replication stress owing to transformation by E6 and E7 oncoproteins. Thus, a rational combination with Rad51 and Wee1 inhibitors holds promise as a novel way to achieve synthetic lethal therapy, particularly in high-grade HPV-positive HNSCC.
EGFR is a transmembrane tyrosine kinase receptor that is commonly overexpressed in HNSCC. EGFR signaling results in resistance to radiochemotherapy at least partially due to the increased DDR capacity. Evidence has shown EGFR signaling promotes DSB repair through PARP1 mediated mechanisms (Myllynen et al., 2015). Consistently, combined inhibition of EGFR and PARP1 enhances radiation effects in terms of persistent DNA damage, induction of senescence, apoptosis and clonogenic cell death, and tumor growth control in mouse xenografts (Frederick et al., 2020). EGFR inhibitor, cetuximab, is the only FDA approved targeted therapy for both HPV-positive and HPV-negative HNSCC. By binding to the ligand-binding domain of EGFR, cetuximab sensitizes HNSCC cells to radiation therapy and improves survival in patients with locally advanced HNSCC (Krishnamurthy et al., 2022).
As a downstream protein of EGFR, phosphatidylinositol 3-kinase (PI3K) is involved in cancer cell proliferation and DNA damage resistance. PI3K/Akt/mTOR signaling pathway is frequently altered in HNSCC and overstimulated with poor prognosis following genotoxic therapies. Schötz et al. found that the PI3K/Akt/mTOR pathway inhibitor BEZ235 sensitizes HPV-negative and HPV-positive HNSCC cells to radiation by suppressing the function of NHEJ (Schotz et al., 2020). However, Glorieux et al. reported that neither a selective pan-class 1 PI3K inhibitor BKM120, nor a dual PI3K-mTOR inhibitor Apitolisib, promoted the radiosensitivity of HPV-negative and HPV-positive HNSCC cells (Glorieux et al., 2020). The inconsistent results are presumably due to the differential off-target effects of these inhibitors, leading to the altered capacity of DDR.
As an important DDR gene, PARP1 represents an attractive therapeutic target for drug development. Several PARP inhibitors have been routinely used in clinical practice to treat BRCA1/2 mutated breast and ovarian cancers. For HNSCC, Weaver et al. found that HPV-positive cells were deficient in DSB repair due to impaired DNA-PKcs and BRCA2, two key genes in NHEJ and HR respectively, resulting in decreased cell survival in vitro and a tumor growth delay in vivo when treated with PARP inhibitor veliparib (Weaver et al., 2015). Moreover, inhibition of PARP1 during radiation significantly increases radiosensitivity of various cancer types (Liu et al., 2018a, 2018b; Weaver et al., 2015). Treatment with PARP inhibitor niraparib enhanced the sensitivity of HPV-positive and HPV-negative HNSCC cells to both photon and proton radiation (Wang et al., 2020). Furthermore, combined inhibition of both PARP1 and XPF promoted cisplatin effects on HPV-negative HNSCC in vitro and in vivo (Zuo et al., 2023). In addition, inhibition of both PARP1 and CHK1/2 gave rise to promising efficacy against HPV-positive HNSCC (Molkentine et al., 2021). The effects of niraparib alone or in combination with cell cycle checkpoint abrogating drugs targeting CHK1 (MK-8776) or Wee1 (MK-1775) were assessed on HNSCC with validated HPV status, where the researchers found radiosensitization with these inhibitors was exhibited in an HPV dependent manner. HPV-positive cells were radiosensitized by MK-8776 and niraparib, while HPV-negative cells were radiosensitized by MK-1775 and niraparib (Molkentine et al., 2021). For HPV-positive HNSCC, combined inhibition of PARP1 and the intra-S/G2 checkpoints achieved effective radiosensitization (Hintelmann et al., 2021), although CHK1 inhibitor alone or in combination with Wee1 inhibitor could cause similar effects (Busch et al., 2017).
Potential ways to improve therapeutic response of HPV-negative HNSCC
HPV-induced DDR defects are responsible for favorable therapeutic response in HNSCC. There are many DDR inhibitors available nowadays. How to exploit these inhibitors for creating similar vulnerabilities in HPV-negative tumors constituted a big challenge in HNSCC treatment.
HPV subjugation of TGFβ signaling leading to enhanced sensitivity to genotoxic agents suggested that TGFβ pathway may be a great target for HPV-negative HNSCC (; b, b; Liu et al., 2021). In these studies, the authors found that loss of TGFβ signaling compromises the DSB repair pathways HR and NHEJ through downregulating the kinase activity of ATM (Kirshner et al., 2006) and inhibits expression of BRCA1(Martinez-Ruiz et al., 2016), and that pharmaceutical inhibition of TGFβ signaling in HPV-negative HNSCC cells replicates the DDR defects exhibited by HPV-positive cells and tumors showing increased sensitivity to radiation or cisplatin (Liu et al., 2018a, 2018b).
As a pleiotropic cytokine, TGFβ not only plays a critical role in regulating the intrinsic cell response (e.g., DDR) to cancer treatment, but also is involved in tumor progression through modifying the tumor microenvironment, suppressing the immune response, promoting metastasis, and vascular remodeling (Liu et al., 2022a, 2022b). Therefore, TGFβ signaling has long been a hot target attracting a concerted effort to develop inhibitors for therapeutic benefit. Studies revealed that higher TGFβ signaling activity (Liu et al., 2018a, 2018b) and more cancer-promoting M2 macrophages infiltration (Fu et al., 2020) in HPV-negative HNSCC comparing to HPV-positive tumors. TGFβ is an inducer for macrophage polarization to M2, while M2 macrophage could induce radiation resistance of HNSCC by releasing HB-EGF and TGFβ to activate DDR pathways (Fu et al., 2020). Thus, targeting TGFβ and /or M2 macrophages would be an efficient way of treating HPV-negative HNSCC. As several TGFβ inhibitors are clinically available, the translational utility of this information could motivate new approaches for treating poor prognosis HPV-negative HNSCC.
Canonical TGFβ signaling is initiated by binding of TGFβ ligands to TGFβ receptors, which causes phosphorylation of the carboxy-terminal serine residue of SMAD2 (SMAD Family Member 2) or SMAD3 (SMAD Family Member 3). Phospho-SMAD2 or SMAD3 oligomerize with SMAD4 (SMAD Family Member 4), resulting in nuclear translocation for transcriptional activation or repression of target genes (Liu et al., 2022a, 2022b). SMAD4 is the only known common-mediator SMAD (Citro et al., 2022). Citro et al. found that HPV stabilizes SMAD4 to increase the expression of the two main players in the DDR pathway, CHK1 and Rad51, in HPV-positive HNSCC tumors and cell lines (Citro et al., 2022). Moreover, SMAD4 silencing increases the sensitivity of HPV-negative HNSCC cell lines to cisplatin (Liu et al., 2018a, 2018b). Taken together, SMAD4 inhibition with DNA damage therapy in HPV-negative HNSCC is expected to achieve better tumor control.
EGFR is another promising target for the precise treatment of HPV-negative HNSCC. EGFR is overexpressed or mutationally activated in over 80% of HPV-negative HNSCC, while HPV-positive HNSCC expresses relatively less EGFR. In a study on EGFR expression in relation to p16, a reliable surrogate marker for HPV infection, both total and nuclear EGFR levels were significantly higher in p16-negative tumors compared to p16-positive tumors (Husain et al., 2012). EGFR activation is strongly indicative of an increased resistance to radiochemotherapy (Alsahafi et al., 2021), suggesting HPV-negative HNSCC may benefit more from EGFR inhibition, although further characterization of EGFR ligand abundance in HPV-negative tumors may be needed for effective treatment with inhibitory EGFR antibodies (Huang et al., 2021). Furthermore, EGFR becomes activated upon radiation, and then translocates into the cell nucleus, where it binds to DNA-PKcs and Ku70 to initiate DNA repair, or indirectly activates PI3K/AKT-dependent phosphorylation of DNA-PKcs, resulting in enhanced DSB repair (Alsahafi et al., 2021; Myllynen et al., 2015). Inhibition of this process may promote radiosensitivity in HPV-negative HNSCC. In 2006, Bonner et al. showed EGFR inhibition by monoclonal antibody cetuximab, in combination with radiotherapy significantly increased HNSCC patient survival, and since then cetuximab has been the only FDA approved targeted drug for the treatment of metastatic HNSCC (Bonner et al., 2006). Unfortunately, whether EGFR blockade with cetuximab performs better in HPV-negative HNSCCs is still unknown.
A recent study revealed that XPF, a DNA endonuclease gene involved in FA pathway, is a promising therapeutic target for cisplatin-refractory HPV-negative HNSCC. We firstly identified a FA-like phenotype in HPV-positive HNSCC cells which showed decreased XPF expression with prolonged G2-M cell cycle arrest and aberrant chromosome formation upon cisplatin treatment. Pharmaceutical inhibition of XPF in HPV-negative HNSCC recapitulated these effects with increased alt-EJ activity. Consistent with these findings, combined inhibition of XPF and alt-EJ further enhanced cisplatin sensitivity in HPV-negative HNSCC in vitro and in vivo (Zuo et al., 2023).
Conclusions
Based on the up-to-date evidence in literature, development of HPV-dependent therapies constitutes an attractive strategy to achieve optimal treatment outcomes for both HPV-positive and HPV-negative HNSCC. HPV is a reliable prognostic biomarker in HNSCC (Ang et al., 2010; Liu et al., 2018a, 2018b; Lohaus et al., 2014; Zech et al., 2022). By exploring the molecular mechanisms in HPV-induced DDR defects, a de-intensified treatment approach for patients with HPV-positive HNSCC can be established, ensuring therapeutic efficacy while reducing toxicity. Importantly, the weakness revealed in HPV-positive HNSCC may shed light on new therapeutic strategies to create similar vulnerabilities and better treatment modality for the poor prognosis HPV-negative HNSCC. We speculate that integrating TGFβ, EGFR and XPF inhibitors into the standard care of HPV-negative HNSCC represents an exciting and promising study field, and development of new therapeutics targeting the alt-EJ pathway worth more research efforts. Results from well-designed clinical trials evaluating these targets are eagerly awaited.
Data availability
The data generated in the present study may be requested from the corresponding author (malin2021@szu.edu.cn).
References
Alsahafi, E. N., Thavaraj, S., Sarvestani, N., Novoplansky, O., Elkabets, M., Ayaz, B., Tavassoli, M., & Legends, M. F. (2021). EGFR overexpression increases radiotherapy response in HPV-positive head and neck cancer through inhibition of DNA damage repair and HPV E6 downregulation. Cancer Letters, 498, 80–97. https://doi.org/10.1016/j.canlet.2020.10.035
Ang, K. K., Harris, J., Wheeler, R., Weber, R., Rosenthal, D. I., Nguyen-Tan, P. F., Westra, W. H., Chung, C. H., Jordan, R. C., Lu, C., Kim, H., Axelrod, R., Silverman, C. C., Redmond, K. P., & Gillison, M. L. (2010). Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine, 363(1), 24–35. https://doi.org/10.1056/NEJMoa0912217
Basukala, O., & Banks, L. (2021). The not-so-good, the bad and the ugly: HPV E5, E6 and E7 oncoproteins in the orchestration of carcinogenesis. Viruses, 13(10), 1892. https://doi.org/10.3390/v13101892
Bonner, J. A., Harari, P. M., Giralt, J., Azarnia, N., Shin, D. M., Cohen, R. B., Jones, C. U., Sur, R., Raben, D., Jassem, J., Ove, R., Kies, M. S., Baselga, J., Youssoufian, H., Amellal, N., Rowinsky, E. K., & Ang, K. K. (2006). Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New England Journal of Medicine, 354(6), 567–578. https://doi.org/10.1056/NEJMoa053422
Busch, C. J., Kroger, M. S., Jensen, J., Kriegs, M., Gatzemeier, F., Petersen, C., Munscher, A., Rothkamm, K., & Rieckmann, T. (2017). G2-checkpoint targeting and radiosensitization of HPV/p16-positive HNSCC cells through the inhibition of Chk1 and Wee1. Radiotherapy and Oncology, 122(2), 260–266. https://doi.org/10.1016/j.radonc.2016.11.017
Castellsague, X., Alemany, L., Quer, M., Halec, G., Quiros, B., Tous, S., Clavero, O., Alos, L., Biegner, T., Szafarowski, T., Alejo, M., Holzinger, D., Cadena, E., Claros, E., Hall, G., Laco, J., Poljak, M., Benevolo, M., Kasamatsu, E., … Neck Cancer Study G. (2016). HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. Journal of National Cancer Institute, 108(6), djv403. https://doi.org/10.1093/jnci/djv403
Citro, S., Miccolo, C., Medda, A., Ghiani, L., Tagliabue, M., Ansarin, M., & Chiocca, S. (2022). HPV-mediated regulation of SMAD4 modulates the DNA damage response in head and neck cancer. Journal of Experimental & Clinical Cancer Research, 41(1), 59. https://doi.org/10.1186/s13046-022-02258-9
de Martel, C., Plummer, M., Vignat, J., & Franceschi, S. (2017). Worldwide burden of cancer attributable to HPV by site, country and HPV type. International Journal of Cancer, 141(4), 664–670. https://doi.org/10.1002/ijc.30716
Deans, A. J., & West, S. C. (2011). DNA interstrand crosslink repair and cancer. Nature Reviews Cancer, 11(7), 467–480. https://doi.org/10.1038/nrc3088
Dok, R., Glorieux, M., Bamps, M., & Nuyts, S. (2021). Effect of ATR inhibition in RT response of HPV-negative and HPV-positive head and neck cancers. International Journal of Molecular Sciences, 22(4), 1504. https://doi.org/10.3390/ijms22041504
Dok, R., Kalev, P., Van Limbergen, E. J., Asbagh, L. A., Vazquez, I., Hauben, E., Sablina, A., & Nuyts, S. (2014). p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Research, 74(6), 1739–1751. https://doi.org/10.1158/0008-5472.CAN-13-2479
Doorbar, J., Quint, W., Banks, L., Bravo, I. G., Stoler, M., Broker, T. R., & Stanley, M. A. (2012). The biology and life-cycle of human papillomaviruses. Vaccine, 30(Suppl 5), F55–F70. https://doi.org/10.1016/j.vaccine.2012.06.083
Fabbrizi, M. R., & Parsons, J. L. (2020). Radiotherapy and the cellular DNA damage response: Current and future perspectives on head and neck cancer treatment. Cancer Drug Resistance, 3(4), 775–790. https://doi.org/10.20517/cdr.2020.49
Faulhaber, E. M., Jost, T., Symank, J., Scheper, J., Burkel, F., Fietkau, R., Hecht, M., & Distel, L. V. (2021). Kinase inhibitors of DNA-PK, ATM and ATR in combination with ionizing radiation can increase tumor cell death in HNSCC cells while sparing normal tissue cells. Genes (basel), 12(6), 925. https://doi.org/10.3390/genes12060925
Fishel, R. (2015). Mismatch repair. Journal of Biological Chemistry, 290(44), 26395–26403. https://doi.org/10.1074/jbc.R115.660142
Frederick, B. A., Gupta, R., Atilano-Roque, A., Su, T. T., & Raben, D. (2020). Combined EGFR1 and PARP1 inhibition enhances the effect of radiation in head and neck squamous cell carcinoma models. Radiation Research, 194(5), 519–531. https://doi.org/10.1667/RR15480.1
Fu, E., Liu, T., Yu, S., Chen, X., Song, L., Lou, H., Ma, F., Zhang, S., Hussain, S., Guo, J., Sun, J., Yu, P., Mao, X., & Wei, L. (2020). M2 macrophages reduce the radiosensitivity of head and neck cancer by releasing HB-EGF. Oncology Reports, 44(2), 698–710. https://doi.org/10.3892/or.2020.7628
Gabani, P., Lin, A. J., Barnes, J., Oppelt, P., Adkins, D. R., Rich, J. T., Zevallos, J. P., Daly, M. D., Gay, H. A., & Thorstad, W. L. (2019). Radiation therapy dose de-escalation compared to standard dose radiation therapy in definitive treatment of HPV-positive oropharyngeal squamous cell carcinoma. Radiotherapy and Oncology, 134, 81–88. https://doi.org/10.1016/j.radonc.2019.01.016
Glorieux, M., Dok, R., & Nuyts, S. (2020). The influence of PI3K inhibition on the radiotherapy response of head and neck cancer cells. Science and Reports, 10(1), 16208. https://doi.org/10.1038/s41598-020-73249-z
Grundy, G. J., & Parsons, J. L. (2020). Base excision repair and its implications to cancer therapy. Essays in Biochemistry, 64(5), 831–843. https://doi.org/10.1042/EBC20200013
Guix, I., Liu, Q., Pujana, M. A., Ha, P., Piulats, J., Linares, I., Guedea, F., Mao, J. H., Lazar, A., Chapman, J., Yom, S. S., Ashworth, A., & Barcellos-Hoff, M. H. (2022). Validation of anti-correlated TGFbeta signaling and alternative end-joining DNA repair signatures that predict response to genotoxic cancer therapy. Clinical Cancer Research. https://doi.org/10.1158/1078-0432.CCR-21-2846
Hintelmann, K., Berenz, T., Kriegs, M., Christiansen, S., Gatzemeier, F., Struve, N., Petersen, C., Betz, C., Rothkamm, K., Oetting, A., & Rieckmann, T. (2021). Dual inhibition of PARP and the intra-S/G2 cell cycle checkpoints results in highly effective radiosensitization of HPV-positive HNSCC cells. Frontiers in Oncology, 11, 683688. https://doi.org/10.3389/fonc.2021.683688
Hu, C., Bugbee, T., Gamez, M., & Wallace, N. A. (2020). Beta human papillomavirus 8E6 attenuates non-homologous end joining by hindering DNA-PKcs activity. Cancers (basel), 12(9), 2356. https://doi.org/10.3390/cancers12092356
Huang, C., Chen, L., Savage, S. R., Eguez, R. V., Dou, Y., Li, Y., da Veiga, L. F., Jaehnig, E. J., Lei, J. T., Wen, B., Schnaubelt, M., Krug, K., Song, X., Cieslik, M., Chang, H. Y., Wyczalkowski, M. A., Li, K., Colaprico, A., Li, Q. K., … Clinical Proteomic Tumor Analysis C. (2021). Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell, 39(3), 361-379 e316. https://doi.org/10.1016/j.ccell.2020.12.007
Huang, Z., Chen, Y., Chen, R., Zhou, B., Wang, Y., Hong, L., Wang, Y., Wang, J., Xu, X., Huang, Z., & Chen, W. (2022). HPV enhances HNSCC chemosensitization by inhibiting SERPINB3 expression to disrupt the Fanconi anemia pathway. Adv Sci (Weinh), 10(1), e2202437. https://doi.org/10.1002/advs.202202437
Husain, H., Psyrri, A., Markovic, A., Rampias, T., Pectasides, E., Wang, H., Slebos, R., Yarbrough, W. G., Burtness, B., & Chung, C. H. (2012). Nuclear epidermal growth factor receptor and p16 expression in head and neck squamous cell carcinoma. The Laryngoscope, 122(12), 2762–2768. https://doi.org/10.1002/lary.23647
Karukonda, P., Odhiambo, D., & Mowery, Y. M. (2022). Pharmacologic inhibition of ataxia telangiectasia and Rad3-related (ATR) in the treatment of head and neck squamous cell carcinoma. Molecular Carcinogenesis, 61(2), 225–238. https://doi.org/10.1002/mc.23384
Keam, B., Machiels, J. P., Kim, H. R., Licitra, L., Golusinski, W., Gregoire, V., Lee, Y. G., Belka, C., Guo, Y., Rajappa, S. J., Tahara, M., Azrif, M., Ang, M. K., Yang, M. H., Wang, C. H., Ng, Q. S., Wan Zamaniah, W. I., Kiyota, N., Babu, S., … Pentheroudakis, G. (2021). Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck. ESMO Open, 6(6), 100309. https://doi.org/10.1016/j.esmoop.2021.100309
Khanal, S., & Galloway, D. A. (2019). High-risk human papillomavirus oncogenes disrupt the Fanconi anemia DNA repair pathway by impairing localization and de-ubiquitination of FancD2. PLoS Pathogens, 15(2), e1007442. https://doi.org/10.1371/journal.ppat.1007442
Kimple, R. J., Smith, M. A., Blitzer, G. C., Torres, A. D., Martin, J. A., Yang, R. Z., Peet, C. R., Lorenz, L. D., Nickel, K. P., Klingelhutz, A. J., Lambert, P. F., & Harari, P. M. (2013). Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Research, 73(15), 4791–4800. https://doi.org/10.1158/0008-5472.CAN-13-0587
Kirshner, J., Jobling, M. F., Pajares, M. J., Ravani, S. A., Glick, A. B., Lavin, M. J., Koslov, S., Shiloh, Y., & Barcellos-Hoff, M. H. (2006). Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Research, 66(22), 10861–10869. https://doi.org/10.1158/0008-5472.CAN-06-2565
Kitamura, N., Sento, S., Yoshizawa, Y., Sasabe, E., Kudo, Y., & Yamamoto, T. (2020). Current trends and future prospects of molecular targeted therapy in head and neck squamous cell carcinoma. International Journal of Molecular Sciences, 22(1), 240. https://doi.org/10.3390/ijms22010240
Kocher, S., Zech, H. B., Krug, L., Gatzemeier, F., Christiansen, S., Meyer, F., Rietow, R., Struve, N., Mansour, W. Y., Kriegs, M., Petersen, C., Betz, C., Rothkamm, K., & Rieckmann, T. (2022). A lack of effectiveness in the ATM-orchestrated DNA damage response contributes to the DNA repair defect of HPV-positive head and neck cancer cells. Frontiers in Oncology, 12, 765968. https://doi.org/10.3389/fonc.2022.765968
Krishnamurthy, S., Ahmed, I., Bhise, R., Mohanti, B. K., Sharma, A., Rieckmann, T., Paterson, C., & Bonomo, P. (2022). The dogma of cetuximab and radiotherapy in head and neck cancer—A dawn to dusk journey. Clinical and Translational Radiation Oncology, 34, 75–81. https://doi.org/10.1016/j.ctro.2022.03.009
Leeman, J. E., Li, Y., Bell, A., Hussain, S. S., Majumdar, R., Rong-Mullins, X., Blecua, P., Damerla, R., Narang, H., Ravindran, P. T., Lee, N. Y., Riaz, N., Powell, S. N., & Higginson, D. S. (2019). Human papillomavirus 16 promotes microhomology-mediated end-joining. Proceedings of the National Academy of Sciences of the United States of America, 116(43), 21573–21579. https://doi.org/10.1073/pnas.1906120116
Leemans, C. R., Braakhuis, B. J., & Brakenhoff, R. H. (2011). The molecular biology of head and neck cancer. Nature Reviews Cancer, 11(1), 9–22. https://doi.org/10.1038/nrc2982
Leonard, B. C., Lee, E. D., Bhola, N. E., Li, H., Sogaard, K. K., Bakkenist, C. J., Grandis, J. R., & Johnson, D. E. (2019). ATR inhibition sensitizes HPV(−) and HPV(+) head and neck squamous cell carcinoma to cisplatin. Oral Oncology, 95, 35–42. https://doi.org/10.1016/j.oraloncology.2019.05.028
Li, G. M. (2008). Mechanisms and functions of DNA mismatch repair. Cell Research, 18, 85–98. https://doi.org/10.1038/cr.2007.115
Lilja-Fischer, J. K., Ulhoi, B. P., Alsner, J., Stougaard, M., Thomsen, M. S., Busk, M., Lassen, P., Steiniche, T., Nielsen, V. E., & Overgaard, J. (2019). Characterization and radiosensitivity of HPV-related oropharyngeal squamous cell carcinoma patient-derived xenografts. Acta Oncologica, 58(10), 1489–1494. https://doi.org/10.1080/0284186X.2019.1660802
Lindemann, A., Patel, A. A., Tang, L., Tanaka, N., Gleber-Netto, F. O., Bartels, M. D., Wang, L., McGrail, D. J., Lin, S. Y., Frank, S. J., Frederick, M. J., Myers, J. N., & Osman, A. A. (2021). Combined inhibition of Rad51 and Wee1 enhances cell killing in HNSCC through induction of apoptosis associated with excessive DNA damage and replication stress. Molecular Cancer Therapeutics, 20(7), 1257–1269. https://doi.org/10.1158/1535-7163.MCT-20-0252
Liu, Q., Chen, G., Moore, J., Guix, I., Placantonakis, D., & Barcellos-Hoff, M. H. (2022a). Exploiting canonical TGFbeta signaling in cancer treatment. Molecular Cancer Therapeutics, 21(1), 16–24. https://doi.org/10.1158/1535-7163.MCT-20-0891
Liu, Q., Gheorghiu, L., Drumm, M., Clayman, R., Eidelman, A., Wszolek, M. F., Olumi, A., Feldman, A., Wang, M., Marcar, L., Citrin, D. E., Wu, C. L., Benes, C. H., Efstathiou, J. A., & Willers, H. (2018a). PARP-1 inhibition with or without ionizing radiation confers reactive oxygen species-mediated cytotoxicity preferentially to cancer cells with mutant TP53. Oncogene, 37(21), 2793–2805. https://doi.org/10.1038/s41388-018-0130-6
Liu, Q., Lopez, K., Murnane, J., Humphrey, T., & Barcellos-Hoff, M. H. (2019). Misrepair in context: TGFbeta regulation of DNA repair. Frontiers in Oncology, 9, 799. https://doi.org/10.3389/fonc.2019.00799
Liu, Q., Ma, L., Jones, T., Palomero, L., Pujana, M. A., Martinez-Ruiz, H., Ha, P. K., Murnane, J., Cuartas, I., Seoane, J., Baumann, M., Linge, A., & Barcellos-Hoff, M. H. (2018b). Subjugation of TGFbeta signaling by human papilloma virus in head and neck squamous cell carcinoma shifts DNA repair from homologous recombination to alternative end joining. Clinical Cancer Research, 24(23), 6001–6014. https://doi.org/10.1158/1078-0432.CCR-18-1346
Liu, Q., Palomero, L., Moore, J., Guix, I., Espin, R., Aytes, A., Mao, J. H., Paulovich, A. G., Whiteaker, J. R., Ivey, R. G., Iliakis, G., Luo, D., Chalmers, A. J., Murnane, J., Pujana, M. A., & Barcellos-Hoff, M. H. (2021). Loss of TGFbeta signaling increases alternative end-joining DNA repair that sensitizes to genotoxic therapies across cancer types. Science Translational Medicine, 13(580), eabc4465. https://doi.org/10.1126/scitranslmed.abc4465
Liu, T., Ma, L., Song, L., Yan, B., Zhang, S., Wang, B., Zuo, N., Sun, X., Deng, Y., Ren, Q., Li, Y., Zhou, J., Liu, Q., & Wei, L. (2022b). CENPM upregulation by E5 oncoprotein of human papillomavirus promotes radiosensitivity in head and neck squamous cell carcinoma. Oral Oncology, 129, 105858. https://doi.org/10.1016/j.oraloncology.2022.105858
Lohaus, F., Linge, A., Tinhofer, I., Budach, V., Gkika, E., Stuschke, M., Balermpas, P., Rodel, C., Avlar, M., Grosu, A. L., Abdollahi, A., Debus, J., Bayer, C., Belka, C., Pigorsch, S., Combs, S. E., Monnich, D., Zips, D., von Neubeck, C., … Dktk, R. O. G. (2014). HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiotherapy and Oncology, 113(3), 317–323. https://doi.org/10.1016/j.radonc.2014.11.011
Martinez-Ruiz, H., Illa-Bochaca, I., Omene, C., Hanniford, D., Liu, Q., Hernando, E., & Barcellos-Hoff, M. H. (2016). A TGFbeta-miR-182-BRCA1 axis controls the mammary differentiation hierarchy. Science Signal, 9(457), ra118. https://doi.org/10.1126/scisignal.aaf5402
Maufort, J. P., Shai, A., Pitot, H. C., & Lambert, P. F. (2010). A role for HPV16 E5 in cervical carcinogenesis. Cancer Research, 70(7), 2924–2931. https://doi.org/10.1158/0008-5472.CAN-09-3436
McBride, A. A. (2022). Human papillomaviruses: diversity, infection and host interactions. Nature Reviews Microbiology, 20(2), 95–108. https://doi.org/10.1038/s41579-021-00617-5
Molkentine, D. P., Molkentine, J. M., Bridges, K. A., Valdecanas, D. R., Dhawan, A., Bahri, R., Hefner, A. J., Kumar, M., Yang, L., Abdelhakiem, M., Pifer, P. M., Sandulache, V., Sheth, A., Beadle, B. M., Thames, H. D., Mason, K. A., Pickering, C. R., Meyn, R. E., & Skinner, H. D. (2022). p16 represses DNA damage repair via a novel ubiquitin-dependent signaling cascade. Cancer Research, 82(5), 916–928. https://doi.org/10.1158/0008-5472.CAN-21-2101
Molkentine, J. M., Molkentine, D. P., Bridges, K. A., Xie, T., Yang, L., Sheth, A., Heffernan, T. P., Clump, D. A., Faust, A. Z., Ferris, R. L., Myers, J. N., Frederick, M. J., Mason, K. A., Meyn, R. E., Pickering, C. R., & Skinner, H. D. (2021). Targeting DNA damage response in head and neck cancers through abrogation of cell cycle checkpoints. International Journal of Radiation Biology, 97(8), 1121–1128. https://doi.org/10.1080/09553002.2020.1730014
Myllynen, L., Kwiatkowski, M., Gleissner, L., Riepen, B., Hoffer, K., Wurlitzer, M., Petersen, C., Dikomey, E., Rothkamm, K., Schluter, H., & Kriegs, M. (2015). Quantitative proteomics unveiled: regulation of DNA double strand break repair by EGFR involves PARP1. Radiotherapy and Oncology, 116(3), 423–430. https://doi.org/10.1016/j.radonc.2015.09.018
Parfenov, M., Pedamallu, C. S., Gehlenborg, N., Freeman, S. S., Danilova, L., Bristow, C. A., Lee, S., Hadjipanayis, A. G., Ivanova, E. V., Wilkerson, M. D., Protopopov, A., Yang, L., Seth, S., Song, X., Tang, J., Ren, X., Zhang, J., Pantazi, A., Santoso, N., … Cancer Genome Atlas N. (2014). Characterization of HPV and host genome interactions in primary head and neck cancers. Proceedings of the National Academy of Sciences of the United States of America, 111(43), 15544–15549. https://doi.org/10.1073/pnas.1416074111
Patterson-Fortin, J., & D’Andrea, A. D. (2020). Exploiting the microhomology-mediated end-joining pathway in cancer therapy. Cancer Research, 80(21), 4593–4600. https://doi.org/10.1158/0008-5472.CAN-20-1672
Schotz, U., Balzer, V., Brandt, F. W., Ziemann, F., Subtil, F. S. B., Rieckmann, T., Kocher, S., Engenhart-Cabillic, R., Dikomey, E., Wittig, A., & Arenz, A. (2020). Dual PI3K/mTOR inhibitor NVP-BEZ235 enhances radiosensitivity of head and neck squamous cell carcinoma (HNSCC) cell lines due to suppressed double-strand break (DSB) repair by non-homologous end joining. Cancers (basel), 12(2), 467. https://doi.org/10.3390/cancers12020467
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Tomaic, V. (2016). Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers (basel), 8(10), 95. https://doi.org/10.3390/cancers8100095
Vitti, E. T., Kacperek, A., & Parsons, J. L. (2020). Targeting DNA double-strand break repair enhances radiosensitivity of HPV-positive and HPV-negative head and neck squamous cell carcinoma to photons and protons. Cancers (basel), 12(6), 1490. https://doi.org/10.3390/cancers12061490
Wang, L., Cao, J., Wang, X., Lin, E., Wang, Z., Li, Y., Li, Y., Chen, M., Wang, X., Jiang, B., Zhang, R., Sahoo, N., Zhang, X., Zhu, X. R., Myers, J. N., & Frank, S. J. (2020). Proton and photon radiosensitization effects of niraparib, a PARP-1/-2 inhibitor, on human head and neck cancer cells. Head and Neck, 42(9), 2244–2256. https://doi.org/10.1002/hed.26155
Weaver, A. N., Cooper, T. S., Rodriguez, M., Trummell, H. Q., Bonner, J. A., Rosenthal, E. L., & Yang, E. S. (2015). DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget, 6(29), 26995–27007. https://doi.org/10.18632/oncotarget.4863
Wu, P., Nielsen, T. E., & Clausen, M. H. (2015). FDA-approved small-molecule kinase inhibitors. Trends in Pharmacological Sciences, 36(7), 422–439. https://doi.org/10.1016/j.tips.2015.04.005
Zech, H. B., Berger, J., Mansour, W. Y., Nordquist, L., von Bargen, C. M., Bussmann, L., Oetting, A., Christiansen, S., Mockelmann, N., Bottcher, A., Busch, C. J., Petersen, C., Betz, C., Rothkamm, K., Kriegs, M., Kocher, S., & Rieckmann, T. (2022). Patient derived ex vivo tissue slice cultures demonstrate a profound DNA double-strand break repair defect in HPV-positive oropharyngeal head and neck cancer. Radiotherapy and Oncology, 168, 138–146. https://doi.org/10.1016/j.radonc.2022.01.017
Zeng, L., Nikolaev, A., Xing, C., Della Manna, D. L., & Yang, E. S. (2020). CHK1/2 inhibitor prexasertib suppresses NOTCH signaling and enhances cytotoxicity of cisplatin and radiation in head and neck squamous cell carcinoma. Molecular Cancer Therapeutics, 19(6), 1279–1288. https://doi.org/10.1158/1535-7163.MCT-19-0946
Zuo, N., Ma, L., Liu, T., Hu, W., Luo, Y., Meng, H., Ren, Q., Deng, Y., Wei, L., & Liu, Q. (2023). Human papillomavirus associated XPF deficiency increases alternative end joining and cisplatin sensitivity in head and neck squamous cell carcinoma. Oral Oncology, 140, 106367. https://doi.org/10.1016/j.oraloncology.2023.106367
Funding
National Natural Science Foundation of China, 82073007, Qi Liu, 82203967, Lin Ma, 82373212, Qi Liu, Basic and Applied Basic Research Foundation of Guangdong Province, 2023A1515011945, Lin Ma, Shenzhen Research and Development Program, JCYJ20210324131607019, Lanlan Wei.
Author information
Qi Liu and Nan Zuo have contributed equally to this paper.
Authors and Affiliations
Department of Stomatology, Shenzhen University General Hospital, Shenzhen University, Shenzhen, China
Qi Liu, Xinghan Li, Yongqiang Deng & Lin Ma
International Cancer Center, Shenzhen University School of Medicine, Shenzhen, China
Qi Liu
Oral Research Center, Qingdao Municipal Hospital, Qingdao, Shandong, China
Nan Zuo
Department of Stomatology, The First Hospital, Harbin Medical University, Harbin, China
Nan Zuo & Lanlan Wei
Institute of Stomatological Research, Shenzhen University, Shenzhen, China
Xinghan Li, Yongqiang Deng & Lin Ma
National Clinical Research Center for Infectious Diseases, The Third People’s Hospital of Shenzhen, The Second Hospital Affiliated to Southern University of Science and Technology, Shenzhen, China
Lanlan Wei
Corresponding authors
Correspondence to Lanlan Wei or Lin Ma.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Zuo, N., Li, X. et al. Novel insights into DNA damage repair defects in HPV-positive head and neck squamous cell carcinoma: from the molecular basis to therapeutic opportunities. GENOME INSTAB. DIS. 4, 255–265 (2023). https://doi.org/10.1007/s42764-023-00109-1
Received19 July 2023
Revised22 September 2023
Accepted24 September 2023
Published11 October 2023
Issue DateOctober 2023
DOIhttps://doi.org/10.1007/s42764-023-00109-1
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable link







用户登录
还没有账号?
立即注册