The role of protein arginine methylation 5 in DNA damage repair and cancer therapy
Review Article
Published: 08 November 2023
Qikun Gao, Ziyi Liu, Jinyang Liu, Xuyang Yan, Junfei Dai, Zixuan Zhang, Rongxiao Li, Shiva Basnet & Changzheng Du
Abstract
Protein arginine methylation, a post-translational modification (PTM), is fundamental in regulating protein function and stability. Among the nine protein methyl transferases (PRMT), PRMT5 plays a critical role in promoting oncogenic processes including tumor proliferation, invasiveness, immune escape and DNA damage repair through different signaling pathways. It is also a target in cancer therapy, with numerous inhibitors in clinical trial. In this review, we focus on the biological functions of PRMT5 in DNA damage repair and maintenance of genome stability in cancer, and summarize the development advance of PRMT5 inhibitors in cancer therapy.
Introduction
Post-translational modification (PTM) is a fundamental protein regulation that realizes or abolishes the function of a protein. Methylation modification on arginine is a common PTM in mammals, catalyzed by the protein arginine methyltransferases (PRMTs) family, which includes nine members from PRMT1 to PRMT9 (Hwang et al., 2021). The methyl groups provided by S-adenosylmethionine (SAM) are transferred by PRMTs to the guanidinium nitrogen atoms of the arginine residue, forming various types of methylated arginine in the substrate protein (Wang et al., 2018). PRMTs are classified into three types depending on the type of methylation modification they catalyze (Fig. 1). PRMT5, a member of the type II PRMT, which consists of 637 amino acids, is gaining increasing attention due to its critical role in cancer and other diseases (Kim & Ronai, 2020; Motolani et al., 2021). PRMT5 catalyzes mono- and symmetric dimethylation modifications on arginine. Its substrate specificity is highly dependent on RGG/RG sequences (PRMT5 target spots), especially the arginine residue, both sides of which are linked to glycine (Gly-Arg-Gly sequence) (Musiani et al., 2019) (Fig. 2). PRMT5 substrates include both histone and non-histone proteins. For histone substrates, particularly the N-terminal histone tails such as H2AR3, H3R2, H3R8, and H4R3, symmetric dimethylation of the aforementioned PRMT5-modified arginines recruits different readers to activate or suppress gene transcription (Kim & Ronai, 2020); for non-histone substrate proteins, methylation of the arginines induce functional alteration in the substrates involving different signaling pathways.
Fig. 1
Classification of PRMT family
Fig. 2
Substrate specificity of PRMT5
PRMT5 has complicated biological function in cancer cells and plays a critical role in many important cellular processes such as transcription, splicing, translation, metabolism, signal transduction and DNA damage repair (DDR) (Yuan et al., 2021). PRMT5 is not only a star molecule in oncogenesis research, but also a valuable target for cancer therapy with a series of inhibitors in clinical trials or approved by the FDA (Wu et al., 2021). Here, we focused on the role of PRMT5 in responding to DNA damage and maintaining genome stability to better understand its potential in cancer research and therapy.
The role of PRMT5 in DNA damage repair and genome stability maintenance
PRMT5 plays a fundamental role in DDR by multiple pathways. By epigenetic regulation, PRMT5 promotes the expression of DDR genes through histone arginine methylation. In prostate cancer, PRMT5 cooperates with partner proteins such as plCln and WDR77 to form an epigenetic activator that upregulates the expression of DDR genes involved in homologous recombination (HR) and non-homologous end joining (NHEJ) pathways, such as KU70/80, RAD51 and BRCA1/2 (Owens et al., 2020). In malignant glioblastoma, PRMT5 promotes the transcription of RNF168, an E3 ligase that activates H2AX and prevents its degradation to enhance the DNA damage response and induce tumor chemoradiation resistance (Du et al., 2019). In addition to double-strand break (DSB) repair, PRMT5 also facilitates inter-cross-link damage (ICL) repair through epigenetic regulation (Du et al., 2021). For example, PRMT5 upregulates H3R2me1 in the promoter regions of FA genes, promoting their expression in cancer cells, resulting in cellular resistance to ICL reagents (Du et al., 2021).
Beside histone arginine methylation, PRMT5 also regulates the expression of DDR proteins by targeting RNA, including splicing and RNA m6A modification. PRMT5 has been shown to play an essential role in maintaining splicing fidelity and genome-level stability by methylating Sm proteins (Sachamitr et al., 2021). In PRMT5-deficient hematopoietic stem cells, gene expression is impaired and DDR-related gene splicing is abnormal, exon skipping and intron retention events increase significantly (Tan et al., 2019). For example, loss of PRMT5 leads to abnormal splicing of TIP60 and SUV4-20H2, both of which play critical roles in chromosome remodeling and DDR protein recruitment during DSB repair (Hamard et al., 2018). PRMT5 also promotes BRCA1 expression after doxorubicin treatment in breast cancer, where PRMT5 attenuates m6A methylation of BRCA1 mRNA, thereby enhancing its stability through methylation of RNA demethylase AlkB homolog 5 (ALKBH5) resulting from translocation of ALKBH5 from the nucleus to the cytoplasm for demethylation of its mRNA substrates including BRCA1 (Wu et al., 2022).
Another regulatory pathway of PRMT5 for genome stability is PTM. Several DDR proteins have been identified as substrates for PRMT5, and their symmetrically dimethylated arginine residues regulate their functions, stability, DNA-binding ability, and interaction with other proteins. A typical example is p53, a central protein in the DDR that maintains genome stability, whose R335 and R337 have been dimethylated by PRMT5 (Hwang et al., 2021). Methylated p53 not only enhances its function in DNA damage response and cell cycle arrest, but also promotes the expression of other DDR genes such as the FA family (Du et al., 2016). Similarly, p53-binding protein 1 (53BP1) is also a substrate of PRMT5, whose R1355 has been methylated by PRMT5 (Hwang et al., 2020). 53BP1 functions as a scaffold protein for the recruitment of DDR proteins to damaged chromatin and promotes NHEJ signaling pathways by limiting end resection after a double-strand break (Clarke et al., 2017). Methylated 53BP1 enhances its proteostasis to facilitate DNA repair through the NHEJ pathway, which could be blocked by Src, a kinase that catalyzes inhibitory phosphorylation of PRMT5 at Y324 (Hwang et al., 2020). In addition to DSB repair, PRMT5 also promotes DNA single-stranded damage (SSB) repair ability by methylating critical members involved in base excision repair (BER) and nucleotide excision repair (NER). PRMT5 enhances the efficiency of BER, by mediating symmetric dimethylation of Flap endonuclease 1 (FEN1) at its R192 (Guo et al., 2010), thereby improving cellular resistance to oxidation-induced DNA damage. For the NER process, PRMT5 dimethylates TDP1 at its R361 and R586, increasing its binding affinity to topoisomerase 1 (Top1) to form a complex that repairs damaged single-stranded DNA (Rehman et al., 2018). Arginine methylation of TDP1 also increases the association of XRCC1 with TDP1 in response to camptothecin, a Top1 inhibitor that causes DNA damage (Rehman et al., 2018).
Above all, PRMT5 modulates DDR and maintains genome stability by multiple pathways. We summarized the targets and regulation mechanism of PRMT5 in DDR process, see Table 1 and Fig. 3.
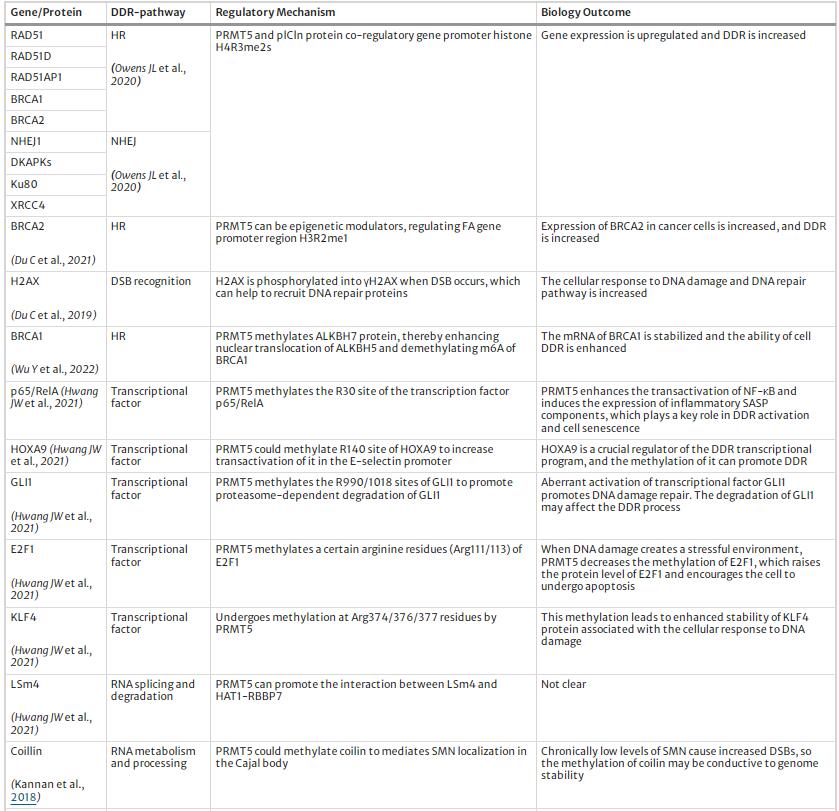
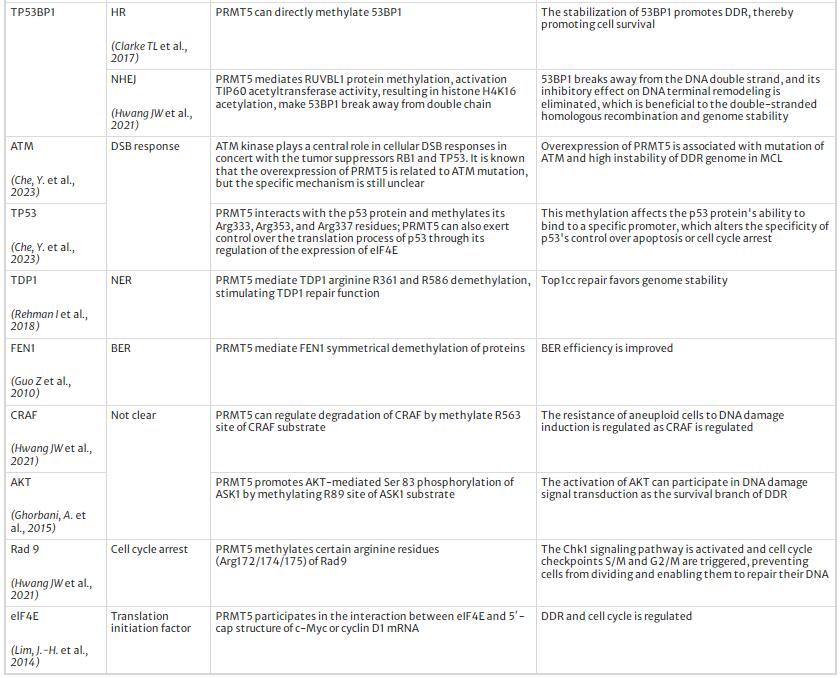
Table 1 The targets and regulatory mechanism of PRMT5 in DDR

Regulatory mechanism of PRMT5 to DNA damage repair
Targeting PRMT5 in cancer therapy
Various PRMT5 inhibitors have been developed and translated into clinical trials (Table 2). Currently, the most commonly used pharmacological approaches for PRMT5 inhibitors include SAM competitive inhibitors, SAM non-competitive inhibitors, and substrate-competitive inhibitors (Fu et al., 2022). In the clinic, PRMT5 inhibitors are classified into two generations according to their target binding site: First-generation inhibitors target the PRMT5 protein directly (e.x. PF0693999) and second-generation inhibitors target the PRMT5-MTA complex (e.x. MRTX1719). Based on the reports from ongoing or finished clinical trials, the therapeutic outcome of PRMT5 inhibitors are encouraging. For example, in a clinical trial organized by MD Anderson Cancer Center, PF-06939999 was proved to dose-dependent and manageable toxicities and 6 mg QD was identified as the recommended monotherapy dose for expansion (NCT03854227). In another multi-center clinical trial, the PRMT5 inhibitor PRT543 showed an excellent anti-inflammation effect and symptom remission rate in selected cancer patients; moreover, a prolonged progression free survival was observed in the patients with refractory myelofibrosis (NCT03886831). With regards to safety, the first-generation inhibitors have been reported to have a high incidence of severe adverse events in clinical trials, including thrombocytopenia, anemia, and gastrointestinal reactions, putting their clinical use at risk (Feustel & Falchook, 2022). In contrast, the second-generation PRMT5 inhibitors were clinically well tolerated and showed acceptable toxicity. The development of second-generation PRMT5 inhibitors is based on the understanding of the enzyme MTAP, which catalyzes the cleavage of methylthioadenosine (MTA) into adenine and 5-methylthioribose-1-phosphate (Bertino et al., 2011). Deletion of MTAP is observed in many cancers, resulting in accumulation of MTA that binds to PRMT5 and attenuates its enzyme activity, leading to lethal synthesis of PRMT5 and MTAP (Kryukov et al., 2016). The second-generation PRMT5 inhibitors mimic MTA to inhibit PRMT5 activity, with optimal clinical effect and acceptable toxicity (Smith et al., 2022). In addition, some marketed drugs and plant-derived natural reagents also have inhibitory effects on PRMT5 (Liu et al., 2022; Prabhu et al., 2023), highlighting their epigenetic and DDR regulatory effects and therapeutic potential in cancer treatment.
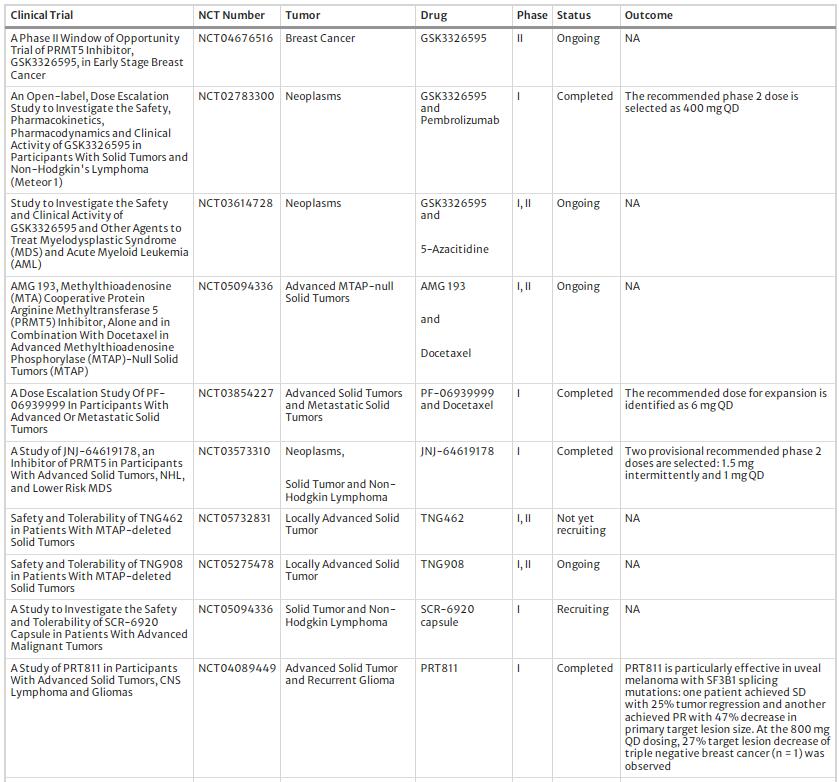
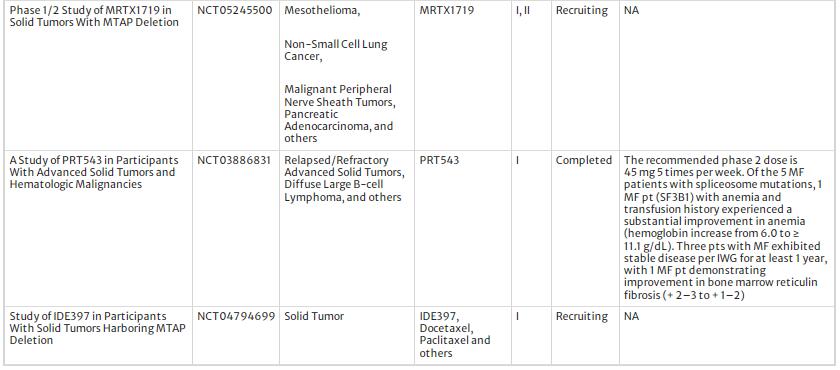
Table 2 The clinical trial of PRMT5 inhibitors
In summary, given the importance of PRMT5 for DDR and maintenance of genome stability, deciphering mechanisms underlying PRMT5 expression, activity, and subcellular localization will advance the development of PRMT5-related therapies in cancer. Although many questions remain about PRMT5, such as the upstream activation pathway of PRMT5 and its shuttle mechanism between the cytoplasm and nucleus, we strongly believe that PRMT5 can be profoundly understood with the help of comprehensive research and will transform cancer therapy in the future with increasingly encouraging clinical trial results.
Data availability
All these data are open assessed.
References
Bertino, J. R., Waud, W. R., Parker, W. B., & Lubin, M. (2011). Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: Current strategies. Cancer Biology & Therapy, 11(7), 627–632.
Che, Y., Liu, Y., Yao, Y., et al. (2023). Exploiting PRMT5 as a target for combination therapy in mantle cell lymphoma characterized by frequent ATM and TP53 mutations. Blood Cancer Journal, 13, 27.
Clarke, T.L/, Sanchez-Bailon. M.P., Chiang, K., Reynolds, J.J., Herrero-Ruiz, J., Bandeiras, T.M., Matias, P.M., Maslen, S.L., Skehel, J.M., Stewart, G.S., Davies C.C. (2017). PRMT5-Dependent methylation of the TIP60 coactivator RUVBL1 is a key regulator of homologous recombination. Mol Cell, 65(5): 900–916 e907.
Du, C., Hansen, L.J., Singh, S.X., Wang, F., Sun, R., Moure, C.J., Roso, K., Greer. P.K., Yan, H., He, Y. (2019). A PRMT5-RNF168-SMURF2 axis controls H2AX proteostasis. Cell Reports, 28(12): 3199–3211 e3195.
Du, C., Li, S. W., Singh, S. X., Roso, K., Sun, M. A., Pirozzi, C. J., Yang, R., Li, J. L., & He, Y. (2021). Epigenetic regulation of fanconi anemia genes implicates PRMT5 blockage as a strategy for tumor chemosensitization. Molecular Cancer Research, 19(12), 2046–2056.
Du, W., Amarachintha, S., Erden, O., Wilson, A., & Pang, Q. (2016). The Fanconi anemia pathway controls oncogenic response in hematopoietic stem and progenitor cells by regulating PRMT5-mediated p53 arginine methylation. Oncotarget, 7(37), 60005–60020.
Feustel, K., & Falchook, G. S. (2022). Protein arginine methyltransferase 5 (PRMT5) inhibitors in oncology clinical trials: A review. J Immunother Precis Oncol, 5(3), 58–67.
Fu, S., Zheng, Q., Zhang, D., Lin, C., Ouyang, L., Zhang, J., & Chen, L. (2022). Medicinal chemistry strategies targeting PRMT5 for cancer therapy. European Journal of Medicinal Chemistry, 244, 114842.
Ghorbani, A., Jeddi-Tehrani, M., Saidpour, A., Safa, M., Bayat, A. A., & Zand, H. (2015). PI3K/AKT and Mdm2 activation are associated with inhibitory effect of cAMP increasing agents on DNA damage-induced cell death in human pre-B NALM-6 cells. Archives of Biochemistry and Biophysics, 566, 58–66.
Guo, Z., Zheng, L., Xu, H., Dai, H., Zhou, M., Pascua, M. R., Chen, Q. M., & Shen, B. (2010). Methylation of FEN1 suppresses nearby phosphorylation and facilitates PCNA binding. Nature Chemical Biology, 6(10), 766–773.
Hamard, P. J., Santiago, G. E., Liu, F., Karl, D. L., Martinez, C., Man, N., Mookhtiar, A. K., Duffort, S., Greenblatt, S., Verdun, R. E., & Nimer, S. D. (2018). PRMT5 regulates DNA repair by controlling the alternative splicing of histone-modifying enzymes. Cell Reports, 24(10), 2643–2657.
Hwang, J. W., Cho, Y., Bae, G. U., Kim, S. N., & Kim, Y. K. (2021). Protein arginine methyltransferases: Promising targets for cancer therapy. Experimental & Molecular Medicine, 53(5), 788–808.
Hwang, J. W., Kim, S. N., Myung, N., Song, D., Han, G., Bae, G. U., Bedford, M. T., & Kim, Y. K. (2020). PRMT5 promotes DNA repair through methylation of 53BP1 and is regulated by Src-mediated phosphorylation. Commun Biol, 3(1), 428.
Kannan, A., Bhatia, K., Branzei, D., & Gangwani, L. (2018). Combined deficiency of Senataxin and DNA-PKcs causes DNA damage accumulation and neurodegeneration in spinal muscular atrophy. Nucleic Acids Research, 46(16), 8326–8346.
Kim, H., & Ronai, Z. A. (2020). PRMT5 function and targeting in cancer. Cell Stress, 4(8), 199–215.
Kryukov, G. V., Wilson, F. H., Ruth, J. R., Paulk, J., Tsherniak, A., Marlow, S. E., Vazquez, F., Weir, B. A., Fitzgerald, M. E., Tanaka, M., Bielski, C. M., Scott, J. M., Dennis, C., Cowley, G. S., Boehm, J. S., Root, D. E., Golub, T. R., Clish, C. B., Bradner, J. E., … Garraway, L. A. (2016). MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science, 351(6278), 1214–1218.
Lim, J. H., Lee, Y. M., Lee, G., et al. (2014). PRMT5 is essential for the eIF4E-mediated 5’-cap dependent translation. Biochemical and Biophysical Research Communications, 452(4), 1016–1021.
Liu, S., Liu, Z., Piao, C., Zhang, Z., Kong, C., Yin, L., & Liu, X. (2022). Flavokawain A is a natural inhibitor of PRMT5 in bladder cancer. Journal of Experimental & Clinical Cancer Research, 41(1), 293.
Motolani, A., Martin, M., Sun, M., Lu, T. (2021). The structure and functions of PRMT5 in human diseases. Life (Basel), 11(10).
Musiani, D., Bok, J., Massignani, E., Wu, L., Tabaglio, T., Ippolito, M.R., Cuomo, A., Ozbek, U., Zorgati. H., Ghoshdastider, U., Robinson, R.C., Guccione, E., Bonaldi, T. (2019). Proteomics profiling of arginine methylation defines PRMT5 substrate specificity. Sci Signal, 12(575).
Owens, J.L., Beketova, E., Liu, S., Tinsley, S.L., Asberry, A.M., Deng, X., Huang, J., Li, C., Wan, J., Hu, C.D. (2020). PRMT5 Cooperates with pICln to Function as a Master Epigenetic Activator of DNA Double-Strand Break Repair Genes. iScience, 23(1): 100750.
Prabhu, L., Martin, M., Chen, L., Demir, O., Jin, J., Huang, X., Motolani, A., Sun, M., Jiang, G., Nakshatri, H., Fishel, M. L., Sun, S., Safa, A., Amaro, R. E., Kelley, M. R., Liu, Y., Zhang, Z. Y., & Lu, T. (2023). Inhibition of PRMT5 by market drugs as a novel cancer therapeutic avenue. Genes Dis, 10(1), 267–283.
Rehman, I., Basu, S. M., Das, S. K., Bhattacharjee, S., Ghosh, A., Pommier, Y., & Das, B. B. (2018). PRMT5-mediated arginine methylation of TDP1 for the repair of topoisomerase I covalent complexes. Nucleic Acids Research, 46(11), 5601–5617.
Sachamitr, P., Ho, J. C., Ciamponi, F. E., Ba-Alawi, W., Coutinho, F. J., Guilhamon, P., Kushida, M. M., Cavalli, F. M. G., Lee, L., Rastegar, N., Vu, V., Sánchez-Osuna, M., Coulombe-Huntington, J., Kanshin, E., Whetstone, H., Durand, M., Thibault, P., Hart, K., Mangos, M., … Dirks, P. B. (2021). PRMT5 inhibition disrupts splicing and stemness in glioblastoma. Nature Communications, 12(1), 979.
Smith, C. R., Aranda, R., Bobinski, T. P., Briere, D. M., Burns, A. C., Christensen, J. G., Clarine, J., Engstrom, L. D., Gunn, R. J., Ivetac, A., Jean-Baptiste, R., Ketcham, J. M., Kobayashi, M., Kuehler, J., Kulyk, S., Lawson, J. D., Moya, K., Olson, P., Rahbaek, L., … Marx, M. A. (2022). Fragment-based discovery of MRTX1719, a synthetic lethal inhibitor of the PRMT5*MTA complex for the treatment of MTAP-deleted cancers. Journal of Medicinal Chemistry, 65(3), 1749–1766.
Tan DQ, Li Y, Yang C, Li J, Tan SH, Chin DWL, Nakamura-Ishizu A, Yang H, Suda T. (2019) PRMT5 Modulates Splicing for Genome Integrity and Preserves Proteostasis of Hematopoietic Stem Cells. Cell Reports, 26(9): 2316–2328 e2316.
Wang, Y., Hu, W., & Yuan, Y. (2018). Protein arginine methyltransferase 5 (PRMT5) as an anticancer target and its inhibitor discovery. Journal of Medicinal Chemistry, 61(21), 9429–9441.
Wu, Q., Schapira, M., Arrowsmith, C. H., & Barsyte-Lovejoy, D. (2021). Protein arginine methylation: From enigmatic functions to therapeutic targeting. Nature Reviews. Drug Discovery, 20(7), 509–530.
Wu, Y., Wang, Z., Han, L., Guo, Z., Yan, B., Guo, L., Zhao, H., Wei, M., Hou, N., Ye, J., Wang, Z., Shi, C., Liu, S., Chen, C., Chen, S., Wang, T., Yi, J., Zhou, J., Yao, L., … Zhang, J. (2022). PRMT5 regulates RNA m6A demethylation for doxorubicin sensitivity in breast cancer. Molecular Therapy, 30(7), 2603–2617.
Yuan, Y., & Nie, H. (2021). Protein arginine methyltransferase 5: A potential cancer therapeutic target. Cellular Oncology (dordrecht), 44(1), 33–44.
Author information
Authors and Affiliations
Department of Biochemistry, Key University Laboratory of Metabolism and Health of Guangdong, Southern University of Science and Technology, 1088 Xueyuan Road, Shenzhen, 518055, China
Qikun Gao, Ziyi Liu, Jinyang Liu, Xuyang Yan, Junfei Dai, Zixuan Zhang, Rongxiao Li, Shiva Basnet & Changzheng Du
Digestive Tumor Center, Southern University of Science and Technology Hospital, Shenzhen, 518055, China
Changzheng Du
Corresponding author
Correspondence to Changzheng Du.
Ethics declarations
Conflict of interest
There is no conflict of interests among the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, Q., Liu, Z., Liu, J. et al. The role of protein arginine methylation 5 in DNA damage repair and cancer therapy. GENOME INSTAB. DIS. (2023). https://doi.org/10.1007/s42764-023-00111-7
Received15 June 2023
Revised05 October 2023
Accepted09 October 2023
Published08 November 2023
DOIhttps://doi.org/10.1007/s42764-023-00111-7
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable link







用户登录
还没有账号?
立即注册