An analysis of gasdermin family of genes in UCEC with respect to malignancy status, mutation percentages and histologic diagnosis
Original Research Paper
Published: 06 May 2024
Caglar Berkel
Volume 5, pages 105–115, (2024)
Abstract
Pyroptosis is a type of programmed lytic cell death mechanism associated with the activation of inflammasomes and inflammatory caspases, proteolytic cleavage of gasdermin proteins (GSDMA-E and PJVK), resulting in the formation of pores in cellular membranes such as plasma membrane and mitochondrial membranes. Here, I show that GSDMC expression was increased, GSDME (DFNA5) and PJVK (DFNB59) expression were decreased in uterine corpus endometrial carcinoma (UCEC) cells compared to normal non-malignant endometrial cells. Total percentage of patients affected by mutations in gasdermin family of genes was the highest in UCEC compared to other cancer types. The highest mutation percentage in UCEC patients among the members of the protein family was observed for GSDME which also showed the most significant difference in the mRNA expression among other family members between tumor and normal samples, possibly pointing to its relatively higher importance in the pathogenesis of UCEC. Gasdermin family of genes (except GSDMA) had higher transcript levels in serous endometrial adenocarcinoma than in endometrioid endometrial adenocarcinoma, demonstrating the histotype-dependent expression of the most of gasdermin family of genes in UCEC. Transcript levels of certain gasdermin family members also differed based on residual tumor status and histologic tumor grade; however, the expression of any gasdermin genes did not change depending on menopause status. This study suggests that a better mechanistic understanding of pyroptotic cell death in uterine corpus endometrial carcinoma might help identify novel therapeutic targets for the management of this gynecological malignancy.
Introduction
Uterine corpus endometrial carcinoma (UCEC) is the second most common gynecological malignancy with high mortality rates. This malignancy has two pathological types: estrogen-dependent endometrioid adenocarcinomas and estrogen-independent serous adenocarcinomas (Huang et al., 2022; Liu & Jing, 2022; Liu et al., 2022; Zhang & Yang, 2021; Zhang et al., 2023). Endometrial carcinoma has also been classified into two main clinicopathological and molecular types: type I is the endometrioid adenocarcinoma (80–90% of all cases), and type II comprises non-endometrioid subtypes including serous, clear cell and undifferentiated carcinomas, as well as carcinosarcoma/malignant-mixed Müllerian tumour (10–20%) (ACOG, 2006; Colombo et al., 2016).
Pyroptosis is a type of lytic pro-inflammatory cell death mechanism induced following the activation of inflammatory caspases (such as caspase-1) by inflammasome complexes including NLRP3 inflammasome. Pyroptotic cell death is mediated by gasdermin (GSDM) proteins (Berkel & Cacan, 2021, 2023; Broz et al., 2020; Liu et al., 2021; Rühl & Broz, 2022; Wei et al., 2022). Gasdermin protein family have six members in humans: GSDMA, GSDMB, GSDMC, GSDMD, GSDME (DFNA5) and PJVK (DFNB59). These proteins are classified as pore-forming effector molecules which induce pyroptosis by disrupting the integrity of the cellular membranes (such as plasma membrane, mitochondrial membranes) upon their activation (Broz et al., 2020; Liu et al., 2021; Miao et al., 2023; Rühl & Broz, 2022). Mechanistically, pyroptosis involves the cleavage of central linker regions (between N- and C-terminal domains) of GSDM proteins by certain caspases, and the release of the GSDM N-terminal (NT) fragment, which targets, for instance, the plasma membrane to form large β-barrel pores of ~ 18 nm in size (this pore formation activity has been verified for all members of the family with the exception of PJVK). These pores formed following the oligomerization of NT gasdermin domains then allow the release of certain pro-inflammatory cytokines such as IL-1β and IL-18 in addition to other cytosolic proteins (cellular alarmins, including ATP, cleaved GSDMs and other cytokines and chemokines) into the extracellular environment (Berkel & Cacan, 2023; Broz et al., 2020; Liu et al., 2021; Rühl & Broz, 2022; Wei et al., 2022). The release of larger molecules such as LDH and HMGB1 also requires the NINJ1-mediated plasma membrane rupture following the formation of gasdermin pores (Berkel & Cacan, 2023; Degen et al., 2023; Kayagaki et al., 2021; Lee et al., 2024; Ramos et al., 2024).
Here, I showed that GSDMC expression is increased, GSDME and PJVK expression are decreased in uterine corpus endometrial carcinoma (UCEC) cells compared to normal endometrial cells. Total percentage of cases affected by mutations in genes encoding gasdermin family of proteins was found to be the highest in UCEC among other cancer types. I also showed that gasdermin family of genes have higher transcript levels in serous endometrial adenocarcinoma than in endometrioid endometrial adenocarcinoma, except for GSDMA. This points to the presence of histological type-dependent expression of the most members of the family in patients with UCEC. Besides, I reported that certain members of the family have differential expression based on residual tumor status and histologic tumor grade. Lastly, I found that the expression of the gasdermin family members in UCEC is independent of menopausal status. Although some previous studies have pointed to the involvement of pyroptotic cell death in patients with UCEC by mostly developing prediction models (Huang et al., 2022; Liu & Jing, 2022; Liu et al., 2022; Zhang & Yang, 2021; Zhang et al., 2023), I here provided novel insights in the context of pyroptotic cell death in uterine corpus endometrial carcinoma. This study suggests that a better mechanistic understanding of pyroptosis in UCEC might help identify novel therapeutic targets for the management of this gynecological malignancy.
Results
GSDMC expression is increased, GSDME and PJVK expression are decreased in uterine corpus endometrial carcinoma compared to normal endometrial cells
First, I compared mRNA levels of gasdermin family of genes (GSDMA-E and PJVK) between tumor samples from patients with uterine corpus endometrial carcinoma (UCEC) and normal control samples from healthy individuals. I found that the expression of GSDMC is increased (p value = 2.2e−08); however, the expression of GSDME and PJVK (Pejvakin) is decreased in tumor samples compared to normal samples (Fig. 1, p values < 2e−16 and = 9.3e−09, respectively). The expression of GSDMA, GSDMB and GSDMD did not significantly differ between tumor and normal samples in the context of UCEC (Fig. 1, p values = 0.65, 0.05 and 0.97, respectively).
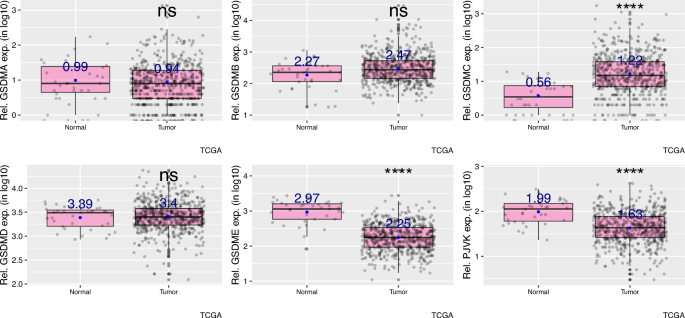
GSDMC expression is increased, GSDME and PJVK expression are decreased in uterine corpus endometrial carcinoma compared to normal endometrial cells. p > 0.05; *p < = 0.05; **p < = 0.01; ***p < = 0.001; ****p < = 0.0001. ns (non-significant), TCGA The Cancer Genome Atlas
Total percentage of cases affected by mutations in genes encoding gasdermin family of proteins is the highest in uterine corpus endometrial carcinoma (UCEC) among other cancer types
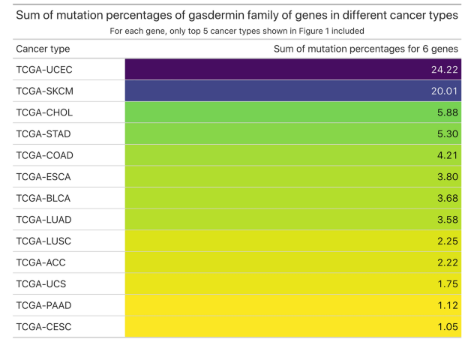
Later, I identified top 5 cancer types for which the percentage of cases affected by mutations in genes encoding gasdermin family of proteins is the highest, for each 6 genes, separately (GSDMA-E and PJVK) (Fig. 2). I found that the percentage of patients affected by mutations in GSDMD, GSDME (DFNA5) or PJVK (DFNB59) is the highest in uterine corpus endometrial carcinoma (UCEC) compared to other cancer types (Fig. 2, last 3 plots). For GSDMA, GSDMB and GSDMC, the percentage of cases affected by mutations in these genes was the second highest in UCEC, following SKCM (skin cutaneous melanoma), among other cancer types (Fig. 2, first 3 plots). When the percentage of cases affected by mutations in these 6 gasdermin genes for the top 5 cancer types (for each gene) were added (given in Fig. 2), UCEC had the highest sum of mutation percentages (24.22%) in gasdermin family of genes, followed by SKCM (20.01%) and CHOL (Cholangiocarcinoma, 5.88%) (Table 1).
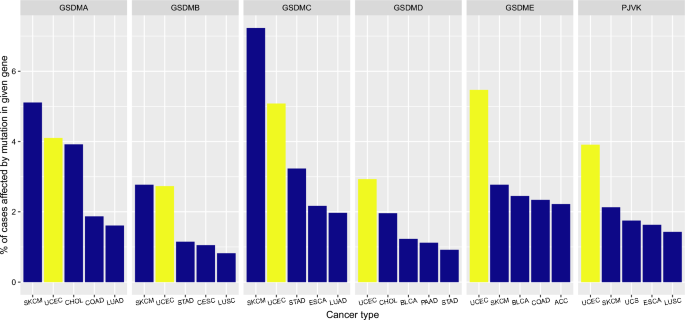
Total percentage of cases affected by mutations in genes encoding gasdermin family of proteins is the highest in uterine corpus endometrial carcinoma (UCEC) among other cancer types. ACC adrenocortical carcinoma, BLCA bladder urothelial carcinoma, CESC cervical squamous cell carcinoma and endocervical adenocarcinoma, CHOL cholangiocarcinoma, COAD colon adenocarcinoma, ESCA esophageal carcinoma, LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, PAAD pancreatic adenocarcinoma, SKCM skin cutaneous melanoma, STAD stomach adenocarcinoma, UCEC uterine corpus endometrial carcinoma, UCS uterine carcinosarcoma
Gasdermin family of genes have higher transcript levels in serous endometrial adenocarcinoma than in endometrioid endometrial adenocarcinoma, except for GSDMA
Later, I showed that the expression of gasdermin family of genes does not depend on tumor status (defined as the condition or state of the tumor at a particular time; “tumor free” vs “with tumor”) (Fig. 3). However, based on histologic diagnosis (i.e. diagnosis of tumor based on the type of tissue, where type is determined based on the microscopic examination of tissue), I found that, for all gasdermin genes except GSDMA, transcript levels are higher in serous endometrial adenocarcinoma than in endometrioid endometrial adenocarcinoma (p values (endometrioid vs serous) are 0.13, 7e−11, 8.5e−06, 0.0016, 2.3e−09 and 0.0089, respectively for GSDMA-E and PJVK) (Fig. 4). In plots, “Mixed” represent mixed serous and endometrioid endometrial adenocarcinoma (Fig. 4). For GSDMB and GSDME, mixed histological type had higher expression than endometrioid histological type of UCEC (Fig. 4; p values are 0.03 and 0.028, respectively).
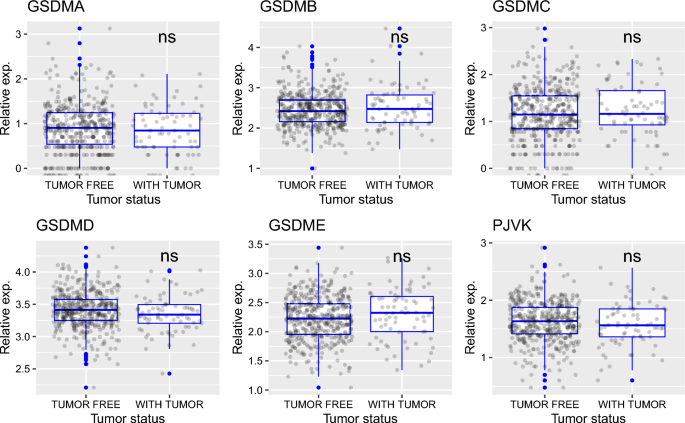
The expression of gasdermin family of genes does not depend on tumor status. Tumor status is defined as the condition or state of the tumor at a particular time: “tumor free” vs “with tumor”. ns non-significant: p > 0.05; *p < = 0.05; **p < = 0.01; ***p < = 0.001; ****p < = 0.0001
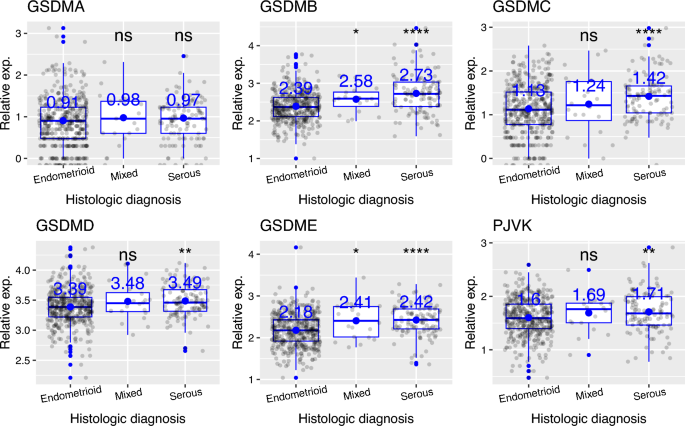
Gasdermin family of genes have higher transcript levels in serous endometrial adenocarcinoma than in endometrioid endometrial adenocarcinoma, except for GSDMA. Serous: serous endometrial adenocarcinoma; Endometrioid: endometrioid endometrial adenocarcinoma; Mixed: mixed serous and endometrioid endometrial adenocarcinoma. ns (non-significant): p > 0.05; *p < = 0.05; **p < = 0.01; ***p < = 0.001; ****p < = 0.0001
Analysis of mRNA expression of gasdermin family of genes based on residual tumor status
Next, I studied the expression of these 6 genes in terms of residual tumor status in uterine corpus endometrial carcinoma (UCEC) samples. Here, R0 is defined as “no residual tumor”, R1 is defined as “microscopic residual tumor (no gross residual disease but positive microscopic margins)”, R2 is defined as “macroscopic residual tumor (grossly visible residual disease)”, and RX is defined as “presence of residual tumor cannot be assessed (the presence of residual tumor or margin status cannot be assessed)”. I found that, for GSDMC and GSDME, R2 samples have higher expression than R1 samples (Fig. 5, p values are 0.023 and 0.035, respectively). GSDMC expression was also higher in R2 samples compared to R0 samples (Fig. 5, p = 0.011) and to R1 samples (Fig. 5). GSDMA and GSDMB transcript levels were found to not change depending on residual tumor status (Fig. 5).
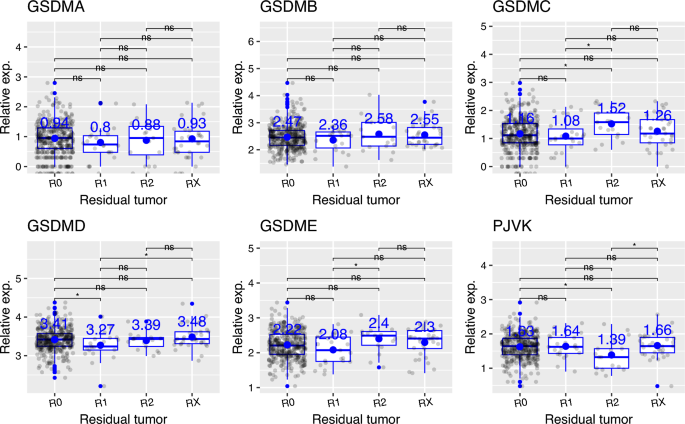
Analysis of mRNA expression of gasdermin family of proteins based on residual tumor. ns (non-significant): p > 0.05; *p < = 0.05; **p < = 0.01; ***p < = 0.001; ****p < = 0.0001
Analysis of the expression of gasdermin family of genes based on histologic tumor grade
Then, I analyzed how the transcript levels of genes encoding gasdermin family of proteins differ depending on the histologic tumor grade in uterine corpus endometrial carcinoma. Firstly, I found that the expression of GSDMC and GSDMD shows an increasing trend from grade 1 (G1) to high grade (G4) (Fig. 6). Here, grade 1 (G1) represents well differentiated (low grade) tumors, grade 2 (G2) represents moderately differentiated (intermediate grade) tumors, grade 3 (G3) represents poorly differentiated (high grade) tumors, and grade 4 (G4) represents undifferentiated (high grade) tumors. For GSDMB, G3 tumors had higher expression than G2 tumors (Fig. 6, p value = 0.039). For GSDMC, G3 tumors had higher expression than G1 tumors. For GSDMD, G3 tumors had increased expression than both G1 and G2 tumors. Similarly, for GSDME, G3 tumors had higher expression than both G1 and G2 tumors (Fig. 6, p values are 0.0011 and 1e−06, respectively). The expression of GSDMA and PJVK did not differ depending on histologic tumor grade (Fig. 6). Lastly, I showed that mRNA expression of genes encoding gasdermin family of proteins does not differ based on menopause status (pre-menopausal vs post-menopausal) (Fig. 7).
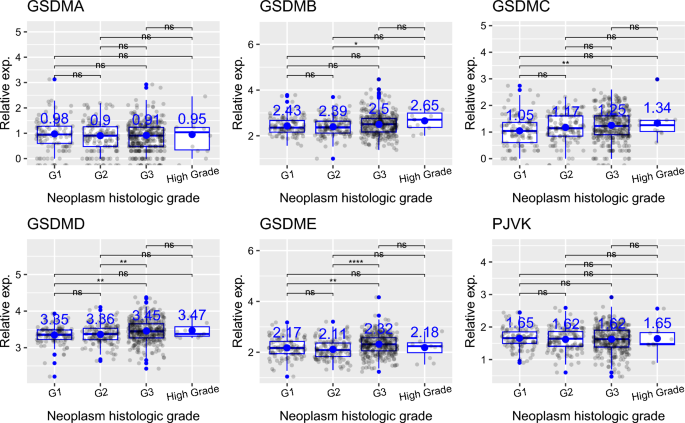
Analysis of the expression of gasdermin family of genes based on histologic tumor grade. ns (non-significant): p > 0.05; *p < = 0.05; **p < = 0.01; ***p < = 0.001; ****p < = 0.0001
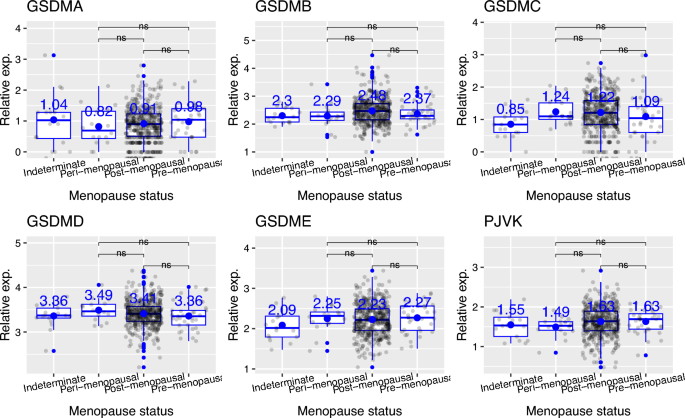
mRNA expression of genes encoding gasdermin family of proteins does not differ based on menopause status (pre-menopausal ve post-menopausal). Post-menopausal: prior bilateral ovariectomy OR > 12 mo since LMP with no prior hysterectomy; Peri-menopausal: 6–12 months since last menstrual period; Indeterminate: neither Pre or Postmenopausal; Pre-menopausal: < 6 months since LMP AND no prior bilateral ovariectomy AND not on estrogen replacement. ns (non-significant): p > 0.05
Discussion
Uterine corpus endometrial carcinoma (UCEC) is one of the most prevalent and lethal gynecologic malignances. Its incidence and mortality rates of patients with UCEC are increasing annually, while its age of onset is decreasing (Sung et al., 2021). Pyroptosis and pore-forming proteins functioning in this cell death mechanism have not been studied in detail in this malignancy; although some risk prediction models have been previously constructed based on pyroptosis-associated genes (Huang et al., 2022; Liu & Jing, 2022; Liu et al., 2022; Zhang & Yang, 2021; Zhang et al., 2023). Here, I first showed that GSDMC expression is increased, GSDME and PJVK expression are decreased in uterine corpus endometrial carcinoma compared to normal endometrial cells. Here, it should be noted that GSDME (DFNA5) and PJVK (DFNB59) belong to the deafness-associated genes (DFN), and their protein sequences cluster more closely together, distant from the other gasdermins (GSDMA–GSDMD) (Broz et al., 2020). Since the expression of both of these genes are decreased in tumor samples from patients with UCEC, unlike the other members of the family, they might have similar or parallel roles in tumor initiation in UCEC. Although other members of the gasdermin family might have both anti-tumor and pro-tumor functions depending on cancer type and context, GSDME and PJVK were reported to have almost always anti-tumor functions (Sarrió et al., 2021). Therefore, their decreased expression in the case of uterine corpus endometrial carcinoma might result in, at least to a certain extent, decreased anti-tumor functionality and increased tumor formation. In contrast, GSMDC has mostly pro-tumor functions in different cancer types (Sarrió et al., 2021); thus, its increased expression observed in tumors of UCEC might contribute to tumor initiation in the case of this gynecologic malignancy. In support, in our two recent studies on ovarian cancer, another gynecologic malignancy, we found that GSDME and PJVK expression are decreased, GSDMC is increased in ovarian tumors compared to normal ovaries, in parallel to what was observed in the present study (Berkel & Cacan, 2021, 2023). Therefore, it can be suggested that changes in the mRNA expression of GSDME, PJVK and GSDMC in the course of tumor formation/initiation in both ovarian cancer and UCEC might lead to similar cellular events, for instance, their insensitivity / resistance to pyroptotic cell death; however, further research is required.
Next, I found that total percentage of patients affected by mutations in gasdermin family of genes (6 genes: GSDMA-E and PJVK) is the highest in UCEC compared to other cancer types. I showed that the percentage of patients affected by mutations in GSDMD, GSDME (DFNA5) or PJVK (DFNB59) is the highest in UCEC compared to other cancer types, and the percentage of patients affected by mutations in GSDMA, GSDMB and GSDMC is the second highest in UCEC, following SKCM (skin cutaneous melanoma), among other cancer types. The highest mutation percentage among the members of the protein family was observed for GSDME, followed by GSDMC. Here, it should be highlighted that the most significant change (tumor vs normal) in the mRNA expression of gasdermin family of genes in UCEC was also observed for GSDME, possibly pointing to its relatively higher importance in the pathogenesis of UCEC compared to other gasdermin family members. Zhang et al. (2020) showed that GSDME suppresses tumour growth by activating anti-tumour immunity in some cancer models; thus, its decreased expression in UCEC similarly might result in only limited supression of tumor growth. They also reported that some cancer-related mutations in GSDME cause its loss of function (Zhang et al., 2020). Since we observed in this study that the highest percentage of mutations in UCEC patients is present in GSDME among other gasdermin family members, and that GSDME has the highest percentage of mutations in UCEC compared to other cancer types, we can state that these mutations in GSDME might lead to loss of its function, possibly leading to inefficient activation of anti-tumor immunity particularly in UCEC. Besides, this relatively higher frequency of mutations in gasdermins in UCEC compared to other cancer types might point to the need for a better mechanistic understanding of this protein family in the context of UCEC.
Later, I showed that, with the exception of GSDMA, all other members of the gasdermin family have higher mRNA expression in serous endometrial adenocarcinoma than in endometrioid endometrial adenocarcinoma. This points to the presence of histotype-dependent expression of gasdermin family in UCEC. Serous endometrial adenocarcinoma, while only accounting for around 10% of all endometrial cancers, is responsible for a disproportionate number of patient deaths (Nakayama et al., 2012). Disease-specific deaths are observed at a higher frequency in serous histological type, because it has higher rates of deep myometrial invasion, more metastatic spread to lymph nodes and peritoneal surfaces compared with endometrioid tumors of endometrial cancer (Ferriss et al., 2021; Hamilton et al., 2006). High expression of the most of gasdermin family members might possibly contribute to worse prognosis in serous endometrial adenocarcinoma compared to endometrioid endometrial adenocarcinoma; however, exact mechanism remains unknown. We have previously shown in another gynecological cancer, ovarian cancer, that high expression of GSDMC and GSDMD is associated with unfavorable progression-free survival (PFS) (Berkel & Cacan, 2021). We have also found that GSDMD transcript levels are higher in serous ovarian cancer than in endometrioid ovarian cancer (Berkel & Cacan, 2021), pointing to the histotype-dependent expression of certain gasdermin family members in gynecological cancers in general, with prognostic implications. However, combined and complex effect of the changes in the expression of all gasdermin family members should be taken into account, considering their context-dependent pro- or anti-tumor activities.
Furthermore, most significant difference in the expression of gasdermin family of genes in terms of histologic grade was observed for GSDME from grade 2 to grade 3 (increased expression; p = 1e−06). Although its expression was decreased in the course of tumor initiation (normal vs tumor), its expression was increased during tumor progression (from low to high grade), pointing to the possibly dual roles of GSDME in tumor initiation and progression in UCEC. For instance, chemotherapeutic drugs-induced pyroptosis mediated by GSDME can promote tumor progression and chemoresistance in the case of pancreatic cancer (Li et al., 2023). Similar scenario might be also in play in the context of UCEC, where increased GSDME levels might lead to tumor progression due to chemotherapeutic drugs-induced pyroptosis after patients are treated with chemotherapy following diagnosis (i.e. following tumor initiation). Further research is required to identify potentially diverse functions of GSDME in the initiation and progression of tumors of UCEC. Some other members of the gasdermin family (GSDMB, GSDMC and GSDMD) also showed generally increased expression from lower to higher tumor grades; however, their contributions to tumor progression need to better characterized.
Combined, I propose that the members of the gasdermin family might have diverse functions in tumor initiation and progression in UCEC, and that the most of these genes show histotype-dependent expression in UCEC, possibly influencing prognosis in patients with UCEC. Moreover, this analysis pointed that GSDME might be relatively more important in the context of UCEC compared to the other members of the family.
Methods
Datasets
In this study, I used TCGA-UCEC transcriptomics dataset which was accessed via ExperimentHub Bioconductor package from GEO dataset with the ID of GSE62944 (Berger et al., 2018; Cancer Genome Atlas Research Network, 2013; Morgan & Shepherd, 2022a, b; Rahman et al., 2015). Total sample size (n) of patients is 554. Sample sizes based on histologic diagnosis are: endometrioid endometrial adenocarcinoma = 401, serous endometrial adenocarcinoma = 112, and mixed serous and endometrioid = 20. Data on mutation percentages for different gasdermin genes were accessed through Genomic Data Commons (GDC) Data Portal of NCI (Berger et al., 2018; Cancer Genome Atlas Research Network, 2013; Grossman et al., 2016). More detail on the transcriptomics and mutation data used can be found elsewhere in the text or in the references (Berger et al., 2018; Cancer Genome Atlas Research Network, 2013; Grossman et al., 2016).
Data analysis and visualization
Data analysis and visualization was completely performed in R programming environment, as previously reported (Berkel, 2024a, b; R Core Team, 2022). Following R/Bioconductor packages were used throughout the analyses: tidyverse (which includes ggplot2 package) (Wickham, 2016; Wickham et al., 2019), readxl (Wickham & Bryan, 2023), ggpubr (Kassambara, 2023), ExperimentHub (Morgan & Shepherd, 2022a, b), AnnotationHub (Morgan & Shepherd, 2022a, b), SummarizedExperiment (Morgan et al., 2022), tidytext (Silge & Robinson, 2016), gt (Iannone et al., 2023), rmarkdown (Allaire et al., 2023) and knitr (Xie, 2023).
When data is not normally distributed, wilcoxon test is used to compare group means. Normality of the expression data was tested using shapiro.test() function in R (R Core Team, 2022). Gene expression count values were shown in log10 scale in y axis of plots. Gene expression data for GSDME was accessed using its alternative name (DFNA5); similarly, gene expression data for PJVK (Pejvakin) was obtained using DFNB59 as a gene name input. All missing data were removed prior to analyses. R code used in the analyses is given as a supplementary document.
Data availability
The data used in this study is publicly available as stated in Methods section.
Abbreviations
ACC:
Adrenocortical carcinoma
BLCA:
Bladder urothelial carcinoma
CESC:
Cervical squamous cell carcinoma and endocervical adenocarcinoma
CHOL:
Cholangiocarcinoma
COAD:
Colon adenocarcinoma
ESCA:
Esophageal carcinoma
GDC:
Genomic data commons
GEO:
Gene expression omnibus
GSDM:
Gasdermin
HMGB1:
High mobility group box protein 1
LUAD:
Lung adenocarcinoma
LUSC:
Lung squamous cell carcinoma
NCI:
National Cancer Institute
PAAD:
Pancreatic adenocarcinoma
PFS:
Progression-free survival
PJVK:
Pejvakin
SKCM:
Skin cutaneous melanoma
STAD:
Stomach adenocarcinoma
TCGA:
The Cancer Genome Atlas
UCEC:
Uterine corpus endometrial carcinoma
UCS:
Uterine carcinosarcoma
References
Allaire, J., Xie, Y., Dervieux, C., McPherson, J., Luraschi, J., Ushey, K., Atkins, A., Wickham, H., Cheng, J., Chang, W., & Iannone, R. (2023). rmarkdown: Dynamic documents for R. R package version 2.21, https://github.com/rstudio/rmarkdown
American College of Obstetricians and Gynecologists. (2006). ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists number 76, October 2006: Postpartum hemorrhage. Obstetrics and Gynecology, 108(4), 1039–1047.
Berger, A. C., Korkut, A., Kanchi, R. S., Hegde, A. M., Lenoir, W., Liu, W., & Moore, R. A. (2018). A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell, 33(4), 690–705.
Berkel, C. (2024a). KIF18A as a potential biomarker to distinguish different breast cancer subtypes based on receptor status. Genome Instability & Disease, 5, 1–8.
Berkel, C. (2024b). Retrospective analysis of transcriptomic differences between triple-negative breast cancer (TNBC) and non-TNBC. European Journal of Biology, 83(1), 1–9.
Berkel, C., & Cacan, E. (2021). Differential expression and copy number variation of gasdermin (GSDM) family members, pore-forming proteins in pyroptosis, in normal and malignant serous ovarian tissue. Inflammation, 44(6), 2203–2216.
Berkel, C., & Cacan, E. (2023). Lower expression of NINJ1 (Ninjurin 1), a mediator of plasma membrane rupture, is associated with advanced disease and worse prognosis in serous ovarian cancer. Immunologic Research, 71(1), 15–28.
Broz, P., Pelegrín, P., & Shao, F. (2020). The gasdermins, a protein family executing cell death and inflammation. Nature Reviews Immunology, 20(3), 143–157.
Cancer Genome Atlas Research Network, Kandoth, C., Schultz, N., Cherniack, A. D., Akbani, R., Liu, Y., Shen, H., Robertson, A. G., Pashtan, I., Shen, R., Benz, C. C., Yau, C., Laird, P. W., Ding, L., Zhang, W., Mills, G. B., Kucherlapati, R., Mardis, E. R., & Levine, D. A. (2013). Integrated genomic characterization of endometrial carcinoma. Nature, 497(7447), 67–73. https://doi.org/10.1038/nature12113
Colombo, N., Creutzberg, C., Amant, F., Bosse, T., González-Martín, A., Ledermann, J., & Sessa, C. (2016). ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. International Journal of Gynecologic Cancer, 26(1), 2–30.
Degen, M., Santos, J. C., Pluhackova, K., Cebrero, G., Ramos, S., Jankevicius, G., Hartenian, E., Guillerm, U., Mari, S. A., Kohl, B., Müller, D. J., Schanda, P., Maier, T., Perez, C., Sieben, C., Broz, P., & Hiller, S. (2023). Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature, 618(7967), 1065–1071. https://doi.org/10.1038/s41586-023-05991-z
Ferriss, J. S., Erickson, B. K., Shih, I. M., & Fader, A. N. (2021). Uterine serous carcinoma: key advances and novel treatment approaches. International Journal of Gynecologic Cancer, 31(8), 1165–1174.
Grossman, R. L., Heath, A. P., Ferretti, V., Varmus, H. E., Lowy, D. R., Kibbe, W. A., & Staudt, L. M. (2016). Toward a shared vision for cancer genomic data. New England Journal of Medicine, 375(12), 1109–1112.
Hamilton, C. A., Cheung, M. K., Osann, K., Chen, L., Teng, N. N., Longacre, T. A., & Chan, J. K. (2006). Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. British Journal of Cancer, 94(5), 642–646.
Huang, X., Li, Y., Li, J., Yang, X., Xiao, J., & Xu, F. (2022). The expression of Pyroptosis-Related gene may influence the occurrence, development, and prognosis of uterine corpus endometrial carcinoma. Frontiers in Oncology, 12, 885114.
Iannone, R., Cheng, J., Schloerke, B., & Hughes, E. (2023). Gt: Easily create presentation-ready display tables. R package version, 9(0).
Kassambara, A. (2023). ggpubr:‘ggplot2’based publication ready plots. R package version 0.6.0.
Kayagaki, N., Kornfeld, O. S., Lee, B. L., Stowe, I. B., O’Rourke, K., Li, Q., Sandoval, W., Yan, D., Kang, J., Xu, M., Zhang, J., Lee, W. P., McKenzie, B. S., Ulas, G., Payandeh, J., Roose-Girma, M., Modrusan, Z., Reja, R., Sagolla, M., … Dixit, V. M. (2021). NINJ1 mediates plasma membrane rupture during lytic cell death. Nature, 591(7848), 131–136. https://doi.org/10.1038/s41586-021-03218-7
Lee, C., Liang, Y., & Li, Y. (2024). Structural and functional insights of NINJ1 in plasma membrane rupture during cell death. Molecular Biomedicine, 5(1), 8. https://doi.org/10.1186/s43556-023-00169-5
Li, S., Yue, M., Xu, H., Zhang, X., Mao, T., Quan, M., Ma, J., Wang, Y., Ge, W., Wang, Y., Xue, S., Shentu, D., Cui, J., & Wang, L. (2023). Chemotherapeutic drugs-induced pyroptosis mediated by gasdermin E promotes the progression and chemoresistance of pancreatic cancer. Cancer Letters, 564, 216206. https://doi.org/10.1016/j.canlet.2023.216206
Liu, J., Geng, R., Ni, S., Cai, L., Yang, S., Shao, F., & Bai, J. (2022). Pyroptosis-related lncRNAs are potential biomarkers for predicting prognoses and immune responses in patients with UCEC. Molecular Therapy-Nucleic Acids, 27, 1036–1055.
Liu, X., Xia, S., Zhang, Z., Wu, H., & Lieberman, J. (2021). Channelling inflammation: Gasdermins in physiology and disease. Nature Reviews Drug Discovery, 20(5), 384–405.
Liu, Z. S., & Jing, C. L. (2022). A novel risk prediction model of pyroptosis-related genes for the prognosis and immunotherapy response of endometrial cancer. European Review for Medical and Pharmacological Sciences, 26(7), 2259–2278.
Miao, R., Jiang, C., Chang, W. Y., Zhang, H., An, J., Ho, F., & Lieberman, J. (2023). Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity, 56(11), 2523–2541.
Morgan M, Obenchain V, Hester J, Pagès H (2022). SummarizedExperiment: SummarizedExperiment container. R package version 1.26.1.
Morgan, M., & Shepherd, L. (2022a). ExperimentHub: Client to access ExperimentHub resources. R package version 2.4.0.
Morgan, M., & Shepherd, L. (2022b). AnnotationHub: Client to access AnnotationHub resources. R package version 3.4.0.
Nakayama, K., Nakayama, N., Ishikawa, M., & Miyazaki, K. (2012). Endometrial serous carcinoma: Its molecular characteristics and histology-specific treatment strategies. Cancers, 4(3), 799–807.
R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 8 Apr 2024.
Rahman, M., Jackson, L. K., Johnson, W. E., Li, D. Y., Bild, A. H., & Piccolo, S. R. (2015). Alternative preprocessing of RNA-Sequencing data in The Cancer Genome Atlas leads to improved analysis results. Bioinformatics, 31(22), 3666–3672.
Ramos, S., Hartenian, E., Santos, J. C., Walch, P., & Broz, P. (2024). NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis. The EMBO Journal, 43(7), 1164–1186. https://doi.org/10.1038/s44318-024-00055-y
Rühl, S., & Broz, P. (2022). Regulation of lytic and non-lytic functions of gasdermin pores. Journal of Molecular Biology, 434(4), 167246.
Sarrió, D., Martínez-Val, J., Molina-Crespo, Á., Sánchez, L., & Moreno-Bueno, G. (2021). The multifaceted roles of gasdermins in cancer biology and oncologic therapies. Biochimica Et Biophysica Acta (BBA)-Reviews on Cancer, 1876(2), 188635.
Silge, J., & Robinson, D. (2016). “tidytext: Text Mining and Analysis Using Tidy Data Principles in R.” JOSS, *1*(3). https://doi.org/10.21105/joss.00037
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249.
Wei, X., Xie, F., Zhou, X., Wu, Y., Yan, H., Liu, T., & Zhang, L. (2022). Role of pyroptosis in inflammation and cancer. Cellular & Molecular Immunology, 19(9), 971–992.
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. Springer.
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D. A., François, R., & Yutani, H. (2019). Welcome to the Tidyverse. Journal of Open Source Software, 4(43), 1686.
Wickham, H., & Bryan, J. (2023). readxl: Read Excel Files. R package version 1.4.2. https://CRAN.R-project.org/package=readxl. Accessed 8 Apr 2024.
Xie, Y. (2023). knitr: A General-Purpose Package for Dynamic Report Generation in R. R package version 1.42
Zhang, C., Bai, J., Yang, Y., Wang, X., Liu, W., Hou, S., & Shao, L. (2023). Construction of prediction model for prognosis of uterine corpus endometrial carcinoma based on pyroptosis gene. Clinical and Translational Oncology, 25(5), 1413–1424.
Zhang, X., & Yang, Q. (2021). A pyroptosis-related gene panel in prognosis prediction and immune microenvironment of human endometrial cancer. Frontiers in Cell and Developmental Biology, 9, 705828.
Zhang, Z., Zhang, Y., Xia, S., Kong, Q., Li, S., Liu, X., Junqueira, C., Meza-Sosa, K. F., Mok, T. M. Y., Ansara, J., Sengupta, S., Yao, Y., Wu, H., & Lieberman, J. (2020). Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature, 579(7799), 415–420. https://doi.org/10.1038/s41586-020-2071-9
Funding
CB is funded by TUBITAK 1001 program.
Author information
Authors and Affiliations
Department of Molecular Biology and Genetics, Tokat Gaziosmanpasa University, Tokat, Turkey
Caglar Berkel
Contributions
CB obtained the data, performed the data analysis and visualization, wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethics approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berkel, C. An analysis of gasdermin family of genes in UCEC with respect to malignancy status, mutation percentages and histologic diagnosis. GENOME INSTAB. DIS. 5, 105–115 (2024). https://doi.org/10.1007/s42764-024-00128-6
Received
Revised
Accepted
Published
Issue Date
DOIhttps://doi.org/10.1007/s42764-024-00128-6
Share this article
Anyone you share the following link with will be able to read this content:








用户登录
还没有账号?
立即注册