MUS81 UFMylation at K400 promotes cell survival in response to camptothecin-induced replication stress
Original Research Paper
Published: 01 July 2024
Qunsong Tan & Xingzhi Xu
Volume 5, pages 154–163, (2024)
Abstract
Camptothecin (CPT) is a widely used chemotherapeutic drug that acts by trapping topoisomerase I (TOP1) on DNA during replication. UFMylation is a ubiquitin-like modification involved in various cellular processes, including DNA double-strand break repair. The role of UFMylation in regulating replication-induced DNA damage within cells, however, is unclear. Through in vivo screening, we ascertained that the structure-specific endonuclease MUS81 is UFMylated. MUS81 is responsible for the progression and restarting of replication forks in human cells. We show that CPT triggered the UFMylation of MUS81 at lysine 400, which in turn prevented its ubiquitination-mediated degradation. Additionally, re-expression of WT MUS81, but not UFMylation defective mutant MUS81(K400R), in MUS81-depleted cells rescued CPT-induced cytotoxicity. Thus, the study revealed a new role for UFMylation in CPT-induced DNA damage, in which MUS81 UFMylation at K400 promotes cancer cell survival by inhibiting MUS81 degradation in response to CPT treatment, thus providing an attractive therapeutic strategy combining UFMylation inhibitors with CPT.
Introduction
Human topoisomerase I (TOP1) is essential for the resolution of topological DNA during genome organization; this enzyme introduces single-strand breaks in a manner mediated by TOP1 cleavage complexes (TOP1cc), which facilitate DNA relaxation and re-ligation (Pommier et al., 2022). The trapping of TOP1cc at DNA by camptothecin (CPT) blocks DNA replication and transcription, resulting in DNA double-strand breaks (DSBs). This process constitutes the main mechanism by which cancer cells are killed (Li &Liu, 2001). When CPT and its derivatives (topotecan and irinotecan) are combined with PARP inhibitors, they act as highly effective cancer-fighting agents in tumor models (Murai et al., 2014). Replication stress at microsatellite repeats also stalls replication forks and causes replication-dependent DSBs, thus representing another route for cancer cell killing (Gadgil et al., 2020).
Numerous pathways can be activated to repair the stalled replication forks induced by TOP1. One pathway involves the removal of the covalently attached TOP1cc via the TDP1-dependent hydrolysis of 3′-phosphodiester bonds near the 3′-DNA end (Pommier, 2009). TOP1 protein can also be hydrolyzed at the N-terminus by the proteases WSS1 and SPRT-like domain or the ubiquitin-dependent proteasome (Duxin et al., 2014; Debethune et al., 2002). The latter process represents a key step in the repair of trapped topoisomerase DNA-protein cross-links. TOP1cc, when conjugated with multi-Ub, was shown to be destroyed via a 26 S-proteasome-dependent mechanism following CPT treatment (Lin et al., 2008; Desai et al., 2001). Ubiquitin-mediated TOP1 degradation can also be triggered by transcription (Desai et al., 2003). RNF4, a small ubiquitin-like modifier (SUMO)-targeted ubiquitin ligase, ubiquitinates SUMO-modified TOP1, which is then targeted by the proteasome for degradation (Sun et al., 2020). Neddylation of the CUL4-RBX1 complex is also required for TOP1cc degradation, indicating that a combination of TOP1 inhibitors and NEDD8-activating enzyme inhibitors can be used to treat colorectal cancers (Sun et al., 2023). Overall, a substantial amount of evidence indicates that protein post-translational modifications (PTMs) are involved in TOP1cc repair.
UFMylation is a post-translational modification that conjugates the ubiquitin-like protein UFM1 to its substrates. The roles of the UFMylation PTM in multiple pathways involved in processes such as regulating the stability, localization, or activity of target proteins have been widely studied (Millrine et al., 2023). Unlike ubiquitin, the two C-terminal amino acids of the pro-form of UFM1 are cleaved to generate the mature form UFM1-ΔC2 for further conjugation (Tatsumi et al., 2010). Then, UFM1-ΔC2 binds to target proteins in a process that involves a UFM1-activating enzyme (E1, UBA5) and a UFM1-conjugating enzyme (E2, UFC1) with or without a UFM1-protein ligase (E3, UFL1) (Benerjee et al., 2020). UFMylation can stabilize p53 protein levels to antagonize tumorigenesis (Liu et al., 2020). Recently, studies have shown that both MRE11 and H4 UFMylation promote ATM activation during DSB repair, which emphasizes the key role of UFMylation in maintaining genome stability (Wang et al., 2019; Qin et al., 2020). How UFMylation functions in replication, and the underlying mechanisms, are unclear however.
MUS81 functions as a nuclease along with its partner EME1/2 to resolve both DSB-formed and reversed replication fork-formed double-Holliday junctions (HJs) (Pepe & West, 2014a, b; Hanada et al., 2007). The MUS81-EME1/2 complex cleaves stalled replication forks and promotes replication fork progression, thus serving as an alternative repair pathway (Regairaz et al., 2011). Studies in yeast showed that TOP1cc repair can occur in the absence of TDP1 via a mechanism that involves MUS81-MMS4 (EME1) (Deng et al., 2005; Liu et al., 2002). More recent studies in mammalian cells indicated that MUS81 involves in removing TOP1cc to promote the progression of stalled replication forks only after the degradation of TOP1 (Regairaz et al., 2011; Marini et al., 2023). Furthermore, the MUS81-EME1/2 complex preserves genome integrity both by cleaving late replication intermediates at chromosomal fracture sites and resolving recombination intermediates to rescue replication forks in the absence of the WRN helicase (Franchitto et al., 2008).
Although the MUS81 complex is involved in TOP1cc repair, little is known about the mechanism by which cells maintain MUS81 protein levels during this process. In this study, we screened for UFM1-conjugating targets involved in maintaining genome stability and found that MUS81 UFMylation at K400 promoted cell survival by counteracting the ubiquitin-mediated degradation of MUS81 in response to CPT treatment. Thus, our results provide evidence that, when used in combination with TOP1 inhibitors, agents targeting UFMylation represent a potential strategy for cancer therapy.
Results
MUS81 is UFMylated at K400
To investigate the role of UFMylation in regulating replication-induced DNA damage within cells, we first used Co-immunoprecipitation (Co-IP) under denature condition to screen for the UFM1-modified substrates of the DNA damage response factors, especially nucleases, involved in the replication stress response pathways. We identified MUS81 as a substrate modified by UFM1, as the band of UFM1-modified MUS81 (higher than 75 kD) was higher than the band of FLAG-tagged MUS81 (lower than 75 kD) (Fig. 1A). To confirm the interaction between MUS81 and UFM1, HEK293T cells were co-transfected with FLAG-tagged MUS81, HA-tagged UFM1-ΔC2 (the mature form of UFM1), with or without MYC-tagged UFC1 (E2) for 48 h, and the proteins were immunoprecipitated under denaturing conditions using an anti-FLAG antibody. Western blot analysis of the immunoprecipitates revealed two specific products (HA-band) of higher molecular weights than those of the FLAG-tagged MUS81 bands (Fig. 1A), indicating that MUS81 was covalently conjugated to poly-UFM1 in the cells.

MUS81 is UFMylated at K400. (A) Purified FLAG-tagged proteins from HEK293T cells co-transfected with FLAG-tagged MUS81 or FLAG-Vector and HA-tagged UFM1-ΔC2 with or without MYC-tagged UFC1 (E2) for 48 h were analyzed by immunoblotting under denaturing conditions. (B) Purified FLAG-tagged proteins from HEK293T cells co-transfected with wild-type MUS81 (MUS81-WT) or MUS81 mutants (K335R, K426R, and K465/467R) with HA-UFM1-ΔC2 and MYC-UFC1 were analyzed by immunoblotting under denaturing conditions. (C) Purified FLAG-tagged proteins from HEK293T cells transfected with FLAG-Vector, FLAG-MUS81-WT or FLAG-MUS81-K400R were analyzed by immunoblotting under non-denaturing conditions. (D) Purified FLAG-tagged proteins from HEK293T cells expressing FLAG-MUS81, HA-UFM1-ΔC2, and E2 were treated with IR (10 Gy) or CPT (1 µM) and collected at the indicated the time-points for analysis by immunoblotting
Full size imageAs MUS81 contains 20 lysine (K) residues, we constructed FLAG-tagged MUS81 mutants to identify the UFMylation site(s) of MUS81 (Fig. 1B). We found that the K335R, K426R, and K465/467R mutants resulted in reduced MUS81 UFMylation compared with MUS81-WT (Fig. 1B). UFMylation assays further confirmed that the K335R, K426R, K465R, and K467R mutants led to reduced MUS81 UFMylation compared with the MUS81-WT (Supplemental Fig. 1A-C). Interestingly, we found that mutation of K400 to arginine (K400R) almost completely inhibited MUS81 UFMylation (Fig. 1B). We thus concluded that K400 is the main UFMylation site of MUS81 (Fig. 1B and Supplemental Fig. 1A-C). As UFL1 is currently the only known E3 ligase for UFM1, we examined whether both MUS81-WT and the K400R mutant interact with UFL1. Co-IP studies demonstrated that both MUS81-WT and the K400R mutant interacted with UFL1 (Fig. 1C), confirming that MUS81 was modified by UFM1 at K400.
To investigate whether MUS81 UFMylation regulates both DSB-induced and replication-induced DNA-damage pathways, we examined the MUS81 UFMylation levels in HEK293T cells expressing FLAG-MUS81, HA-UFM1-ΔC2, and E2 with or without ionizing radiation (IR)-introduced DSBs or CPT-introduced replication stress. Interestingly, UFMylation assays showed that CPT stimulated the UFMylation of MUS81 both 1 h and 2 h after treatment, while IR did not (Fig. 1D), indicating the involvement of MUS81 UFMylation in the CPT-induced replication stress response. Collectively, these results demonstrate that MUS81 is UFMylated at K400, which might in turn regulate its function during the CPT-induced DNA damage response.
UFMylation of MUS81 at K400 does not regulate its endonuclease activity
MUS81 forms a heterodimeric complex with the noncatalytic subunit EME1 or EME2 in vivo (Pepe & West, 2014a, b; Boddy et al., 2001). We thus examined whether MUS81 UFMylation at K400 regulates the association between MUS81 and EME1/2. To do so, we performed co-IP of HEK293T cells co-transfected with HA-tagged EME1 or EME2 and FLAG-tagged MUS81-WT or MUS81-K400R. Western blot analysis showed the MUS81-K400R mutant had similar interaction with both EME1 and EME2 compared with MUS81-WT (Fig. 2A-B).
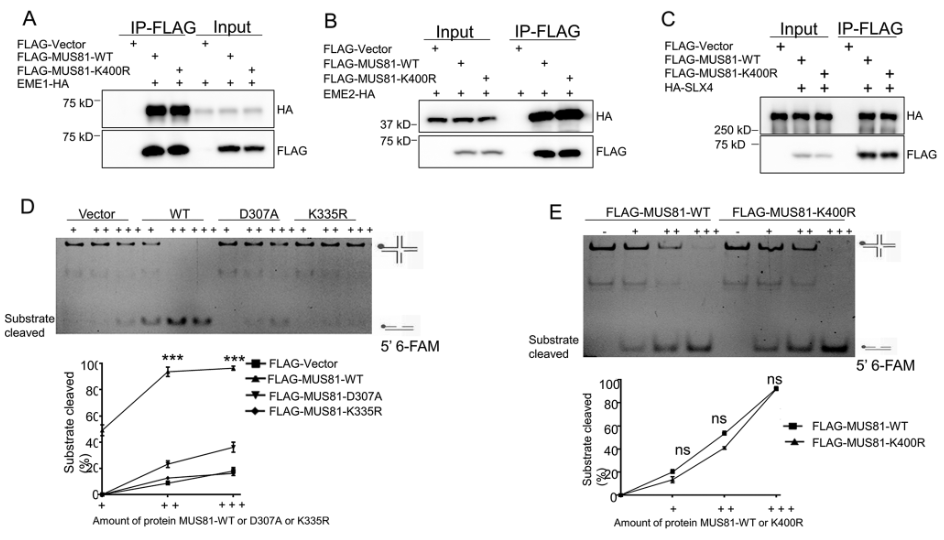
UFMylation of MUS81 at K400 does not regulate its endonuclease activity. (A) Purified FLAG-tagged proteins from HEK293T cells co-transfected with HA-tagged EME1 and FLAG-Vector, FLAG-tagged MUS81-WT, or FLAG-tagged MUS81-K400R for 48 h were analyzed by immunoblotting after IP under non-denaturing conditions. (B) Purified FLAG-tagged proteins from HEK293T cells expressing HA-tagged EME2 and FLAG-Vector, FLAG-MUS81-WT, or FLAG-MUS81-K400R were analyzed by immunoblotting after IP under non-denaturing conditions. (C) Purified FLAG-tagged proteins from HEK293T cells expressing HA-tagged SLX4 and FLAG-Vector, FLAG-MUS81-WT, or FLAG-MUS81-K400R were analyzed by immunoblotting. (D) In vitro endonuclease assay of purified FLAG-tagged proteins from HEK293T expressing HA-tagged EME2 and FLAG-Vector, FLAG-MUS81-WT, FLAG-MUS81-D307A, or FLAG-MUS81-K335R using DNA substrates (nHJs) followed by non-denaturing PAGE; bar graph shows quantification of the data. (E) In vitro endonuclease assay of purified FLAG-tagged proteins from HEK293T expressing MUS81-WT or MUS81-K400R using DNA substrates (nHJs) followed by non-denaturing PAGE; bar graph shows quantification of the data. Data represent the means ± SD (n = 3 experiments). ns, not significant; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
Full size imageAnother interaction between the scaffold protein SLX4 and the MUS81-EME1/2 complex mediates the activation of the SLX1-SLX4, MUS81-EME1, and XPF-ERCC1 tri-nuclease (Wyatt et al., 2017). We therefore investigated whether MUS81 UFMylation determines the interaction between SLX4 and MUS81. Co-IP of HA-tagged SLX4- and FLAG-tagged MUS81-WT- or MUS81-K400R-expressing cells showed that MUS81-WT interacted with SLX4 (Fig. 2C), and this interaction was seen with the MUS81-K400R mutant (Fig. 2C). Thus, the loss of UFMylation at K400 did not disrupt the association between MUS81 and EME1/2 or SLX4.
The MUS81 K400 residue is located within the nuclease activity domain (amino acids (aa) 245–469). We therefore hypothesized that MUS81 nuclease activity is regulated by K400 UFMylation. To test this hypothesis, we purified FLAG-tagged proteins from HEK293T cells expressing HA-tagged EME2 and FLAG-Vector, FLAG-MUS81-WT, FLAG-tagged MUS81-D307A (catalytically inactive mutant), or FLAG-MUS81-K335R. In vitro endonuclease assays using the DNA substrates nicked HJs (nHJs) followed by non-denaturing PAGE showed that MUS81-WT cleaved the nHJs in a concentration-dependent manner, while MUS81-D307A failed to cleave the substrates, even at the highest concentration (Fig. 2D). Further endonuclease assays using FLAG-tagged MUS81-K400R purified from FLAG-tagged MUS81-K400R- and HA-tagged EME2-expressing cells revealed that the cleavage of these substrates by MUS81-K400R was comparable to that mediated by MUS81-WT (Fig. 2E). However, the endonuclease activity of the MUS81-K335R mutant was almost completely lost compared to that of MUS81-WT (Fig. 2D), while there was no significantly change in the nuclease activity of MUS81-K465R, K467R or K524R mutants (Supplemental Fig. 2). These data suggest that MUS81 endonuclease activity is not regulated by K400 UFMylation.
UFMylation at K400 inhibits MUS81 degradation in response to CPT exposure
Our finding that CPT induces MUS81 UFMylation at K400 without limiting its enzymatic activity prompted us to interrogate whether UFMylation at K400 affects MUS81 stability in response to CPT-induced DNA damage. We first compared the half-lives of MUS81-WT and MUS81-K400R proteins in cells by performing cycloheximide (CHX, 15 µg/mL) chase experiments. Western blot analysis showed that the half-life of the MUS81-K400R protein was similar to that of the MUS81-WT protein, and both proteins were fully degraded after 12 h (Fig. 3A).
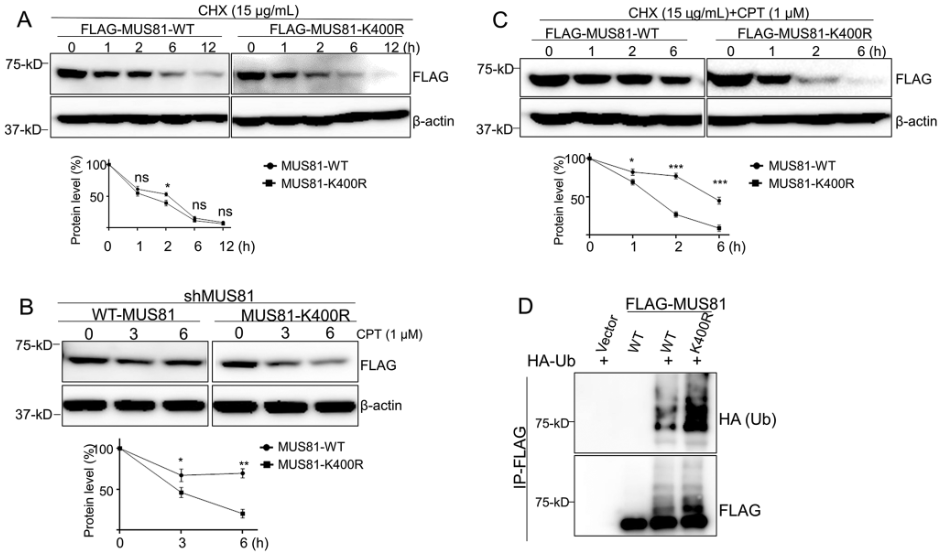
UFMylation at K400 inhibits degradation of MUS81 in response to CPT exposure. (A) Western blot analysis of FLAG-MUS81-WT- and FLAG-MUS81-K400R-expressing cells treated with cycloheximide (CHX, 15 µg/mL) and collected at the indicated time-points. (B) Western blot analysis of FLAG-MUS81-WT- and FLAG-MUS81-K400R-expressing cells treated with cycloheximide (CHX, 15 µg/mL) and CPT (1 µM) and collected at the indicated time-points. (C) Western blot analysis of FLAG-MUS81-WT- and FLAG-MUS81-K400R-expressing cells treated with CPT (1 µM) and collected at the indicated time-points. (D) FLAG-tagged proteins were purified from HEK293T cells co-transfected with HA-tagged Ub and FLAG-Vector, FLAG-MUS81-WT, or FLAG-MUS81-K400R for 36 h. FLAG-Vector, FLAG-MUS81-WT, and FLAG-MUS81-K400R were analyzed by immunoblotting under denaturing conditions. Bar graphs in A-C show protein levels measured using ImageJ. The data represent the means ± SD. ns, not significant; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
Full size imageGiven that CPT stimulates MUS81 degradation, we wanted to test whether UFMylation at K400 maintains MUS81 protein levels in response to CPT treatment. To do so, we constructed MUS81-depleted HCT116 cells by transfecting them with a lentivirus expressing a short-hairpin (sh)RNA targeting the 3’-UTR of the MUS81 gene (Supplemental Fig. 3A). We then stably re-expressed FLAG-tagged MUS81-WT and FLAG-tagged MUS81-K400R in the shMUS81 cells (Supplemental Fig. 3A). A western blot showed that cells with shRNA had markedly reduced MUS81 expression compared with the shControl group cells, and both the FLAG-tagged MUS81-WT and FLAG-tagged MUS81-K400R rescued the expression of MUS81 (Supplemental Fig. 3A). Then, we evaluated the stability of the two proteins MUS81-WT and MUS81-K400R in cells exposed to CPT or cisplatin (CDDP). We observed that approximately 80% of the MUS81-K400R proteins were degraded 6 h after CPT but not CDDP treatment, while only 40% of the MUS81-WT proteins were degraded at this time-point (Fig. 3B and Supplemental Fig. 3B). Furthermore, we found that, while approximately 90% of MSU81-K400R protein was degraded in 2 h, only around 40% of MUS8-WT protein was degraded within this time when cells were treated with both CPT and CHX (Fig. 3C). Thus, the results showed that UFMylation sustained the MUS81-WT protein levels in cells following CPT and CHX treatment, and K400 was required for this effect.
To explore the stability of MUS81 with multiple sites simultaneously mutated, we constructed MUS81-K335/400/524R mutant and performed protein stability assay accordingly. It was found that the stability of MUS81-K335/400/524R mutant exhibited almost identical pattern to that of MUS81-K400R mutant upon CPT treatment (Supplemental Fig. 3C), indicating that CPT-induced MUS81 stability mainly depends on UFMylation at K400.
Protein ubiquitination is a major pathway of protein degradation in cells (Ciechanover, 1998). As such, we examined the cells for evidence of UFMylation and ubiquitination crosstalk. To provide direct evidence that UFMylation at K400 regulates MUS81 stability by inhibiting the ubiquitin-dependent pathway, we compared the ubiquitination levels of MUS81-WT and MUS81-K400R in HEK293T cells co-transfected with HA-tagged Ub and FLAG-MUS81-WT or FLAG-MUS81-K400R for 36 h. After denaturing IP and western blot analyses, we found that the ubiquitination level of MUS81-K400R was markedly higher than that of MUS81-WT (Fig. 3D), indicating that the UFMylation of MUS81 at K400 prevented its ubiquitination. Taken together, these results provide strong evidence that UFMylation at K400 prevents MUS81 degradation via the ubiquitination pathway during the response to DNA damage induced by CPT.
MUS81 UFMylation at K400 promotes HCT-116 cell survival in response to CPT treatment
The stalling of replication forks induced by TOP1cc causes chromosome aberrations and genome instability (Tuduri et al., 2009). Our finding that UFMylation at K400 regulates MUS81 stability in response to CPT treatment prompted us to explore the possibility that UFMylation is connected to cell survival and chemotherapy responses.
We thus tested whether inhibiting UFMylation would reduce cell colony formation in response to CPT treatment. To further investigate the role of MUS81 UFMylation at K400 in chromosome aberrations, we performed a metaphase spread assay in HCT116 cell lines expressing shControl, shMUS81, and MUS81-WT or MUS81-K400R and treated with CPT. We observed significantly more CPT-induced chromosome aberrations in both MUS81-depleted cells (approximately 4 per cell) and MUS81-K400R-expressing cells (approximately 3 per cell) than in the negative control or MUS81-WT-expressing cells (approximately 1 per cell) (Fig. 4A). Our findings demonstrate that MUS81 UFMylation at K400 is essential for genome integrity after CPT treatment.
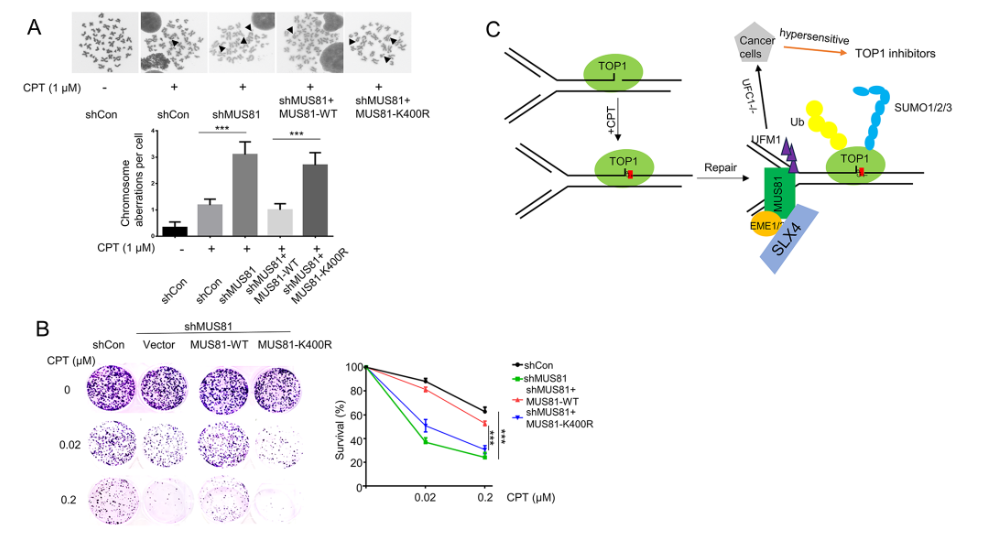
MUS81 UFMylation at K400 promotes HCT-116 cell survival in response to CPT treatment. (A) Chromosome aberration analysis (bottom) and representative images from one of three independent experiments (top) using the indicated cell lines. Genomic instability was measured as metaphase spread in the indicated HCT-116 cell lines treated with CPT (1 µM, 1 h); 50 metaphases were analyzed per condition. (B) Representative images of clonogenic assay cultures (12 days) (left) of cells transfected with shControl, shMUS81, FLAG- MUS81-WT, or FLAG-MUS81-K400R and treated with indicated concentration of CPT for 1 h. (C) Working model depicting the function of MUS81 UFMylation in response to CPT-induced replication stress. TOP1 removes DNA negative supercoiling during the replication process. CPT traps TOP1 in the chromatin and stimulates ubiquitin-mediated degradation of TOP1 to repair CPT-induced DNA damage. MUS81-mediated DNA cleavage recovers CPT-induced stalled replication forks. MUS81 UFMylation counteracts MUS81 ubiquitination and degradation at CPT-induced replication forks. Targeting the UFMylation pathway combined with inhibition of TOP1 is implicated as a novel strategy to enhance cancer therapy efficiency. The data represent the means ± SD (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001)
Full size imageWe next evaluated the viability of shMUS81 HCT116 cells expressing MUS81-WT and MUS81-K400R following CPT treatment (0.02 µM and 0.2 µM) for 1 h. Clonogenic assays showed that MUS81-deficient (~ 30%) and MUS81-K400R (~ 40%) cells were much more sensitive to the low concentration of CPT (0.02 µM) than those expressing shControl (~ 85%) or MUS81-WT (~ 80%) (Fig. 4B). We observed the same results for the clonogenic formation of MUS81-K400R- and MUS81-WT-expressing cells at 0.2 µM CPT (Fig. 4B). Furthermore, we also determined effects of other mutants with decreasing MUS81 UFMylation on cell survival in response to CPT treatment. Our results showed that re-expression of MUS81-WT, MUS81-K465R, or MUS81-K467R, but not MUS81-K335R, rescued CPT-induced decrease of survival of shMUS81 cells, while re-expression of MUS81-524R could partially rescued (Supplemental Fig. 4). We therefore concluded that MUS81 UFMylation at K400 facilitates cell survival in response to CPT treatment.
Discussion
Genomic instability, a hallmark of various cancers, is also the main mechanism by which anti-cancer drugs induce the lethal DNA lesions that lead to a therapeutic response (Reuvers et al., 2020). UFMylation has emerged as an important posttranslational modification that repairs DNA damage to maintain genome integrity. In this study, we uncovered a new mechanism by which UFMylation promotes cell survival by inhibiting the CPT-induced degradation of MUS81 (Fig. 4C). Based on our results, we propose a working model for the function of MUS81 UFMylation at K400 in response to CPT-induced replication stress (Fig. 4C). According to our model, UFL1-mediated MUS81 UFMylation at K400 maintains MUS81 protein levels within cells by inhibiting MUS81 degradation after CPT treatment. MUS81 then promotes the repair of CPT-induced stalled replication and replication fork progression. The loss of MUS81 UFMylation at K400 rendered HCT116 cells more sensitive to CPT treatment, indicating that targeting the UFMylation pathway may serve as a strategy for cancer therapy.
We were interested to note that CPT, but not IR, induced MUS81 UFMylation at K400 (Fig. 1B and D). A 2011 study of HCT116 cells in vitro showed that following CPT treatment, the stalled replication forks induced by TOP1cc are resolved by the action of MUS81 and the EME1/2 heterodimer complex, leading to replication fork progression and cell survival (Regairaz et al., 2011). MUS81 has been shown to contribute to the recovery of stalled replication forks by processing detrimental replication-associated structures into DSBs (Pepe & West, 2014a, b; Van et al., 2020). Although the K400 UFMylation site we identified is located in the nuclease domain of MUS81, the MUS81-K400R mutant did not reduce the MUS81 nuclease activity when compared with MUS81-WT, indicating that UFMylation at K400 may function as an alternative mechanism for regulating the progress of replication forks stalled by CPT treatment. However, we found that the UFMylation level of MUS81-K335R, which is located in the conserved ERKXXXD sequence of the nuclease domain, was also lower than that of MUS81-WT (Fig. 1B). This MUS81-K335R mutation completely abolished MUS81’s nuclease activity (Fig. 2C), indicating that UFMylation might also help to regulate MUS81 nuclease activity to maintain cellular function, as well as having a role in maintaining MUS81 stability in response to CPT. Given that K335 of MUS81 is highly conserved among species in the nuclease domain and thus critical for its endonuclease activity (Pepe & West, 2014a, b), it could be challenging to differentiate if this particular residue, its UFMylation, or other post-translation modifications impact or govern its catalytic activity.
The sequential SUMOylation and ubiquitination of TOP1cc is required for their rapid degradation by the proteasome (Sun et al., 2020). We thus investigated the possibility of a link between the UFMylation of MUS81 and its stability, a phenomenon that has been reported to be necessary for the regulation of p53 protein stability (Liu et al., 2020). Our results showed that UFMylation at K400 counteracted MUS81 ubiquitination and inhibited CPT-induced MUS81 degradation (Fig. 3B-D), thus providing evidence that UFMylation and ubiquitination work together to maintain protein levels. This indicates that using TOP1 inhibitors combined with UFMylation pathway-targeting agents is a potential strategy for cancer therapy.
Our results are consistent with previous reports (Regairaz et al., 2011) that showed MUS81 is essential for genome integrity and cell survival during the CPT-induced replication stress response (Fig. 4A-B). Interestingly, we found that replication-fork-associated chromatin aberrations were significantly lower in cells expressing the MUS81-K400R mutant than those expressing the MUS81-WT (Fig. 4A). Cell viability assays also demonstrated that loss of MUS81 UFMylation at K400 led to the hypersensitivity of cells to CPT treatment (Fig. 4B). Thus, our findings showed that MUS81 UFMylation at K400 is essential for cell survival after CPT treatment, highlighting the potential efficacy of inhibiting MUS81 UFMylation as an approach to improve the anticancer effects of CPT (Fig. 4C).
In conclusion, our study has demonstrated that UFMylation at K400 maintains MUS81 protein levels to promote cell survival and the repair of CPT-induced DNA damage by countering ubiquitin-dependent MUS81 degradation. This mechanism may also be important in other repair pathways, such as interstrand crosslinking, DNA mismatch repair, and translesion DNA synthesis pathways. However, as CPT blocks replication fork progression, it remains unknown whether UFMylation regulates replication fork progression and CPT-induced replication reversal or restart. Further studies are now warranted to explore the specific function of MUS81 UFMylation at K400 in replication processes and how it might be modulated to improve the efficacy of anticancer therapeutic strategies.
Materials and methods
Cell lines
HEK293T and HCT116 cell lines were purchased from ATCC. Cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and incubated in a 37℃ humidified incubator with a 5% CO2 atmosphere.
MUS81-knockdown was achieved in HCT116 cells by lentiviral infection. First, an shRNA targeting the 3’-UTR (sense: 5’-CATTGCAGCTTGGAATCTATT-3’) was cloned into a pLKO.1 vector and transfected in HEK293T cells with packaging plasmids (psPAX2 and pMD2.G) and pLKO.1-shRNA, pLKO.1-shControl, lenti-Blast-FLAG-MUS81-WT, or lenti-Blast-FLAG-MUS81-K400R. Lentiviral particles were harvested at 48 h post-transfection and used to infect HCT116 cells, which were selected using puromycin (2 µg/mL) to generate the shMUS81 cell line. The shMUS81 cell line was then infected with lenti-Blast viruses and selected with Blasticidin (2 µg/mL). The depletion of MUS81 and expression of FLAG-MUS81-WT or FLAG-MUS81-K400R in HeLa cells were confirmed by western blotting.
Plasmids and transfection
The MUS81, EME1, and EME2 sequences were amplified from HEK293T cDNA and cloned into pcDNA3.1-HA, pcDNA3.1-FLAG, or lenti-Blast-FLAG vectors using the pEASY® Basic Seamless Cloning and Assembly kit (TransGen). MUS81 mutations were amplified by PCR with the corresponding primers (K to R mutation), and the products were digested with DpnI for transformation in Trans5α (TransGen); the mutation was confirmed by sequencing. HA-SLX4, HA-UFM1-Δ2, and MYC-UFC1(E2) were kindly provided by Dr. Gong Ya Min (Shenzhen University). Plasmids or siRNAs were transfected into HEK293T or HCT116 cells with PEI reagent or RNAiMAX (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Immunoprecipitation
FLAG- or HA-tagged peptides were transiently expressed in HEK293T cells. For native protein IP, cells were washed twice with cold PBS and lysed with IP buffer (50 mM tris-HCl (pH 7.5), 150 mM NaCl, 0.5% NP-40, 1 mM EDTA) supplemented with a cocktail of phosphatase and proteinase inhibitors for 30 min at 4℃ followed by centrifugation at 12,000 × g for 10 min at 4℃. For IP under denaturing conditions, cells were washed twice with cold PBS and lysed in 5× SDS buffer containing 250 mM Tris-HCl (pH 7.5), 500 mM NaCl, and 5% SDS, and boiled at 100℃ for 30 min. The lysate was then digested with benzonase and diluted 5-fold with RIPA buffer (50 mM Tris (pH 7.4), 150 mM NaCl, 0.5% TritonX-100). The diluted denatured and native lysates were centrifuged at 12,000 × g for 15 min at 4℃ and the FLAG-tagged protein was purified from the soluble lysate by adding anti-FLAG (M2) (MilliporeSigma) beads according to the manufacturer’s instructions. After rotating overnight, the FLAG-M2 beads were washed at least three times with IP buffer and boiled with SDS loading buffer for 10 min before further analysis.
Immunoblotting
Western blotting was performed as described previously. For protein stability assays, cells were incubated with CHX (15 µg/mL) with or without CPT (1 µM) and collected at the indicated time-points. In brief, cells were washed with cold PBS and lysed with RIPA buffer containing a cocktail of phosphatase and proteinase inhibitors (Bimake) for 30 min at 4℃, followed by centrifugation at 12,000 × g for 15 min at 4℃. The total protein concentration was determined using a BCA kit (Thermofisher). Equal amounts of total proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane, which was then blocked by incubation with 5% skimmed milk in PBST (0.2% Tween20 in PBS) at room temperature for 1 h. Subsequently, the membrane was incubated with the following primary detection antibodies: anti-MUS81 (1:1,000, Abclonal), anti-MUS81 (1:1,000, Proteintech), anti-γH2AX (1:1,000, CST), anti-RPA32-S4/S8 (1:1,000, Bethyl), anti-β-actin (1:3,000, Abclonal), anti-FALG (1:2,000, Invitrogen), anti-HA (1: 1000, Proteintech), anti-UFC1 (1:1,000, Proteintech), and anti-UFL1 (1:1,000, Bethyl). After washing with PBST, the membrane was incubated with the indicated second antibody labeled with HRP (Jackson ImmunoResearch). Detection was carried out as per standard procedures.
DNA nuclease assays
5’-FAM-labeled DNA substrates were synthesized, and nHJ substrates were prepared by annealing the following oligonucleotides (5’-3’):
HJ-S1: TATTTCGAACTGCTAATGTGGTCTCCCTGCAGATACGGGTGGACGTCCAA.
HJ-S2: TTGGACGTCCACCCGTATCTGCAGGGTCTGGCCGTGACCATCTTAAGCCG.
HJ-S3: CAGCCTAGGAGATCTGCAATCGTGGGAGACCACATTAGCAGTTCGAAATA.
HJ-S4: CGGCTTAAGATGGTCACGGCCAGAC.
HJ-S5: CCACGATTGCAGATCTCCTAGGCTG.
Nuclease cleavage assays of the indicated amounts of proteins were performed in reactions containing 50 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 50 mM NaCl, 1 mM DTT, glycerol 5%, 0.1 mg/mL BSA, and 25 µM nHJs. The reaction mixtures were incubated at 37℃ for 1 h and stopped by adding 2× stop buffer containing protease K (2 mg/mL) and 200 mM EDTA (pH 8.0) before further incubation at 65℃ for 10 min. The products were then analyzed by 12% native PAGE in 1× TBE (89 mM Tris-base, 89 mM boric acid, and 2 mM EDTA). The DNA gels were imaged using Amersham Imager 600 (GE Healthcare), and cleavage products were quantified by using ImageJ. FLAG-tagged proteins were purified from FLAG-tagged MUS81-WT- or MUS81-K400R-expressing HEK293T cells using FLAG-M2 beads according to the manufacture’s protocol.
Metaphase spread
After treatment with CPT (1 µM) for 1 h, shControl, shMUS81, FLAG-tagged MUS81-WT, or MUS81-K400R cell lines were washed with PBS and cultured in fresh medium at 37℃ for 24 h. Cells were exposed to colchicine (0.1 µg/mL) for an additional 4 h. Cells were subsequently harvested and resuspended in medium containing 75 mM KCl and 10% FBS for 10 min at 37℃, followed by fixation using methanol: acetic acid (3:1) overnight at 4℃. Cells were dropped onto cold slides and air-dried overnight, and then mounted with Prolong Gold Antifade (Invitrogen) containing DAPI. Images were captured under a microscope using a 60×/1.4 oil-immersion objective (NiKon Eclipse Ti2 and Andor Fusion software) and analyzed with ImageJ.
Cell viability
All HCT116 cell lines were seeded into 6-well plates (500 cells/well) and incubated for 24 h at 37℃ before treatment with the indicated dose of CPT for 1 h. The cells were then cultured in fresh medium for 10–14 days. Fixation and staining of colonies were performed using 0.05% methylene blue containing methanol for 30 min at room temperature. The visualized colonies were subsequently counted.
Statistical analysis
Data are presented as the means ± standard deviation (SD), and the data from two groups were analyzed by Student’s t-test using GraphPad Prism software. P-values < 0.05 were considered to indicate statistical significance.
Data availability
All data generated and analyzed in this study are included in this published article and its supplementary information files.
References
Banerjee, S., Kumar, M., & Wiener, R. (2020). Decrypting UFMylation: How proteins are modified with UFM1 biomolecules 10, Artn 144210.3390/Biom10101442.
Boddy, M. N., Gaillard, P. H. L., McDonald, W. H., Shanahan, P., Yates, J. R., & Russell, P. (2001). Mus81-Eme1 are essential components of a Holliday junction resolvase cell 107, 537–548 https://doi.org/10.1016/S0092-8674(01)00536-0
Ciechanover, A. (1998). The ubiquitin-proteasome pathway: On protein death and cell life. Embo J, 17, 7151–7160. https://doi.org/10.1093/emboj/17.24.7151
Article PubMed PubMed Central CAS Google Scholar
Debethune, L., Kohlhagen, G., Grandas, A., & Pommier, Y. (2002). Processing of nucleopeptides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Research, 30, 1198–1204. https://doi.org/10.1093/nar/30.5.1198
Article PubMed PubMed Central CAS Google Scholar
Deng, C., Brown, J. A., You, D., & Brown, J. M. (2005). Multiple endonucleases function to repair covalent topoisomerase I complexes in Saccharomyces cerevisiae. Genetics, 170, 591–600. https://doi.org/10.1534/genetics.104.028795
Article PubMed PubMed Central CAS Google Scholar
Desai, S. D., Li, T. K., Rodriguez-Bauman, A., Rubin, E. H., & Liu, L. F. (2001). Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as a resistance mechanism to camptothecin in tumor cells. Cancer Research, 61, 5926–5932.://WOS:000170194700039.
PubMed CAS Google Scholar
Desai, S. D., Zhang, H., Rodriguez-Bauman, A., Yang, J. M., Wu, X. H., Gounder, M. K., Rubin, E. H., & Liu, L. F. (2003). Transcription-dependent degradation of topoisomerase I-DNA covalent complexes. Molecular and Cellular Biology, 23, 2341–2350. https://doi.org/10.1128/Mcb.23.7.2341-2350.2003
Article PubMed PubMed Central CAS Google Scholar
Duxin, J. P., Dewar, J. M., Yardimci, H., & Walter, J. C. (2014). Repair of a DNA-protein crosslink by replication-coupled proteolysis. Cell, 159, 346–357. https://doi.org/10.1016/j.cell.2014.09.024
Article PubMed PubMed Central CAS Google Scholar
Franchitto, A., Pirzio, L. M., Prosperi, E., Sapora, O., Bignami, M., & Pichierri, P. (2008). Replication fork stalling in WRN-deficient cells is overcome by prompt activation of a MUS81-dependent pathway. Journal of Cell Biology, 183, 241–252. https://doi.org/10.1083/jcb.200803173
Article PubMed PubMed Central CAS Google Scholar
Gadgil, R. Y., Romer, E. J., Goodman, C. C., Rider, S. D., Damewood, F. J., Barthelemy, J. R., Shin-ya, K., Hanenberg, H., & Leffak, M. (2020). Replication stress at microsatellites causes DNA double-strand breaks and break-induced replication. Journal of Biological Chemistry, 295, 15378–15397. https://doi.org/10.1074/jbc.RA120.013495
Article PubMed PubMed Central CAS Google Scholar
Hanada, K., Budzowska, M., Davies, S. L., van Drunen, E., Onizawa, H., Beverloo, H. B., Maas, A., Essers, J., Hickson, I. D., & Kanaar, R. (2007). The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand. DNA Breaks Nat Struct Mol Biol, 14, 1096–1104. https://doi.org/10.1038/nsmb1313
Article PubMed CAS Google Scholar
Li, T. K., & Liu, L. F. (2001). Tumor cell death induced by topoisomerase-targeting. Drugs Annual Review of Pharmacology and Toxicology, 41, 53–77. https://doi.org/10.1146/annurev.pharmtox.41.1.53
Article PubMed Google Scholar
Lin, C. P., Ban, Y., Lyu, Y. L., Desai, S. D., & Liu, L. F. (2008). A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. Journal of Biological Chemistry, 283, 21074–21083. https://doi.org/10.1074/jbc.M803493200
Article PubMed PubMed Central CAS Google Scholar
Liu, C., Pouliot, J. J., & Nash, H. A. (2002). Repair of topoisomerase I covalent complexes in the absence of the tyrosyl-DNA phosphodiesterase Tdp1. Proceedings of the National Academy of Sciences of the United States of America, 99, 14970–14975. https://doi.org/10.1073/pnas.182557199
Article PubMed PubMed Central CAS Google Scholar
Liu, J., Guan, D., Dong, M. G., Yang, J. J., Wei, H. B., Liang, Q., Song, L. Z., Xu, L., Bai, J. J., Liu, C., Mao, J., Zhang, Q., Zhou, J. Z., Wu, X. Y., Wang, M., & Cong, Y. S. (2020). UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nature Cell Biology, 22, 1056–. https://doi.org/10.1038/s41556-020-0559-z
Article PubMed CAS Google Scholar
Marini, V., Nikulenkov, F., Samadder, P., Juul, S., Knudsen, B. R., & Krejci, L. (2023). MUS81 cleaves TOP1-derived lesions and other DNA-protein cross-links. Bmc Biol, 21, ARTN110. https://doi.org/10.1186/s12915-023-01614-1
Article CAS Google Scholar
Millrine, D., Peter, J. J., & Kulathu, Y. (2023). A guide to UFMylation, an emerging posttranslational modification. Febs J, 290, 5040–5056. https://doi.org/10.1111/febs.16730
Article PubMed PubMed Central CAS Google Scholar
Murai, J., Zhang, Y. P., Morris, J., Ji, J. P., Takeda, S., Doroshow, J. H., & Pommier, Y. (2014). Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or Temozolomide based on PARP trapping versus Catalytic Inhibition. Journal of Pharmacology and Experimental Therapeutics, 349, 408–416. https://doi.org/10.1124/jpet.113.210146
Article PubMed PubMed Central CAS Google Scholar
Pepe, A., & West, S. C. (2014). Substrate specificity of the MUS81-EME2 structure selective endonuclease. Nucleic Acids Research, 42, 3833–3845. https://doi.org/10.1093/nar/gkt1333
Article PubMed CAS Google Scholar
Pepe, A., & West, S. C. (2014a). MUS81-EME2 promotes replication fork. Restart Cell Rep, 7, 1048–1055. https://doi.org/10.1016/j.celrep.2014.04.007
Article PubMed CAS Google Scholar
Pommier, Y. (2009). DNA topoisomerase I inhibitors: Chemistry, biology, and interfacial inhibition. Chemical Reviews, 109, 2894–2902. https://doi.org/10.1021/cr900097c
Article PubMed PubMed Central CAS Google Scholar
Pommier, Y., Nussenzweig, A., Takeda, S., & Austin, C. (2022). Human topoisomerases and their roles in genome stability and organization. Nat Rev Mol Cell Bio, 23, 407–427. https://doi.org/10.1038/s41580-022-00452-3
Article CAS Google Scholar
Qin, B., Yu, J., Nowsheen, S., Zhao, F., Wang, L. W., & Lou, Z. K. (2020). STK38 promotes ATM activation by acting as a reader of histone H4 ufmylation Science advances 6, ARTN eaax821410.1126/sciadv.aax8214.
Regairaz, M., Zhang, Y. W., Fu, H., Agama, K. K., Tata, N., Agrawal, S., Aladjem, M. I., & Pommier, Y. (2011). Mus81-mediated DNA cleavage resolves replication forks stalled by topoisomerase I-DNA complexes. The Journal of cell Biology, 195, 739–749. https://doi.org/10.1083/jcb.201104003
Article PubMed PubMed Central CAS Google Scholar
Reuvers, T. G. A., Kanaar, R., & Nonnekens, J. (2020). DNA damage-inducing Anticancer therapies: From Global to Precision damage cancers 12, Artn 209810.3390/Cancers12082098.
Sun, Y., Miller Jenkins, L. M., Su, Y. P., Nitiss, K. C., Nitiss, J. L., & Pommier, Y. (2020). A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Science Advances, 6. https://doi.org/10.1126/sciadv.aba6290
Sun, Y., Baechler, S. A., Zhang, X., Kumar, S., Factor, V. M., Arakawa, Y., Chau, C. H., Okamoto, K., Parikh, A., Walker, B., Su, Y. P., Chen, J., Ting, T., Huang, S. N., Beck, E., Itkin, Z., McKnight, C., Xie, C., Roper, N., & Pommier, Y. (2023). Targeting neddylation sensitizes colorectal cancer to topoisomerase I inhibitors by inactivating the DCAF13-CRL4 ubiquitin ligase complex Nature communications 14, 3762 https://doi.org/10.1038/s41467-023-39374-9
Tatsumi, K., Sou, Y. S., Tada, N., Nakamura, E., Iemura, S., Natsume, T., Kang, S. H., Chung, C. H., Kasahara, M., Kominami, E., Yamamoto, M., Tanaka, & Komatsu, K., M (2010). A novel type of E3 ligase for the Ufm1 conjugation system. Journal of Biological Chemistry, 285, 5417–5427. https://doi.org/10.1074/jbc.M109.036814
Article PubMed CAS Google Scholar
Tuduri, S., Crabbé, L., Conti, C., Tourrière, H., Holtgreve-Grez, H., Jauch, A., Pantesco, V., De Vos, J., Thomas, A., Theillet, C., Pommier, Y., Tazi, J., Coquelle, A., & Pasero, P. (2009). Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nature Cell Biology, 11, 1315–U1125. https://doi.org/10.1038/ncb1984
Article PubMed PubMed Central CAS Google Scholar
van Wietmarschen, N., Sridharan, S., Nathan, W. J., Tubbs, A., Chan, E. M., Callen, E., Wu, W., Belinky, F., Tripathi, V., Wong, N., Foster, K., Noorbakhsh, J., Garimella, K., Cruz-Migoni, A., Sommers, J. A., Huang, Y., Borah, A. A., Smith, J. T., Kalfon, J., & Nussenzweig, A. (2020). Repeat expansions confer WRN dependence in microsatellite-unstable cancers. Nature, 586, 292–298. https://doi.org/10.1038/s41586-020-2769-8
Article PubMed PubMed Central CAS Google Scholar
Wang, Z. F., Gong, Y. M., Peng, B., Shi, R. F., Fan, D., Zhao, H. C., Zhu, M., Zhang, H. X., Lou, Z. K., Zhou, J. W., Zhu, W. G., Cong, Y. S., & Xu, X. Z. (2019). MRE11 UFMylation promotes ATM activation nucleic acids research 47, 4124–4135 https://doi.org/10.1093/nar/gkz110
Wyatt, H. D. M., Laister, R. C., Martin, S. R., Arrowsmith, C. H., & West, S. C. (2017). The SMX DNA repair tri-nuclease Mol Cell 65, 848– https://doi.org/10.1016/j.molcel.2017.01.031
Download references
Acknowledgements
Q.T. and X.X. designed the study, and Q.T. performed laboratory experiments and analyzed the data. Q.T. and X.X. wrote the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (NSFC) grant (32090031).
Author information
Authors and Affiliations
Guangdong Key Laboratory for Genome Stability & Disease Prevention and Carson International Cancer Center, Marshall Laboratory of Biomedical Engineering, Shenzhen University Medical School, Shenzhen, 518060, China
Qunsong Tan & Xingzhi Xu
Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National- Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen, 518060, China
Qunsong Tan
Department of Cell Biology, Shenzhen University Medical School, Shenzhen University, Shenzhen, Guangdong, 518060, China
Xingzhi Xu
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests except that Prof. Xingzhi Xu is the Deputy Editor-in-Chief of the journal "Genome Instability and Disease", and he was not involved in the peer review or the decision making of the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article
Tan, Q., Xu, X. MUS81 UFMylation at K400 promotes cell survival in response to camptothecin-induced replication stress. GENOME INSTAB. DIS. 5, 154–163 (2024). https://doi.org/10.1007/s42764-024-00132-w
Download citation
Received
Revised
Accepted
Published
Issue Date
DOIhttps://doi.org/10.1007/s42764-024-00132-w
Share this article
Anyone you share the following link with will be able to read this content:
Provided by the Springer Nature SharedIt content-sharing initiative
Keywords
UFMylation
MUS81
CPT
Ubiquitination
Cancer therapy








用户登录
还没有账号?
立即注册